DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue
- PMID: 20975052
- PMCID: PMC2974463
- DOI: 10.1212/WNL.0b013e3181f9615d
DQB1*0602 predicts interindividual differences in physiologic sleep, sleepiness, and fatigue
Abstract
Objective: The human leukocyte antigen (HLA) DQB1*0602 allele is closely associated with narcolepsy, a neurologic disorder characterized by excessive daytime sleepiness, fragmented sleep, and shortened REM sleep latency. We evaluated whether DQB1*0602 was a novel marker of interindividual differences by determining its relationship to sleep homeostatic, sleepiness, and cognitive responses to baseline and chronic partial sleep deprivation (PSD) conditions.
Methods: Ninety-two DQB1*0602-negative and 37 DQB1*0602-positive healthy adults participated in a protocol of 2 baseline 10 hours time in bed (TIB) nights followed by 5 consecutive 4 hours TIB nights. DQB1*0602 allelic frequencies did not differ significantly between Caucasians and African Americans.
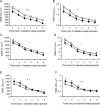
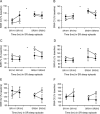
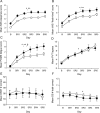
Results: During baseline, although DQB1*0602-positive subjects were subjectively sleepier and more fatigued, they showed greater sleep fragmentation, and decreased sleep homeostatic pressure and differentially sharper declines during the night (measured by non-REM EEG slow-wave energy [SWE]). During PSD, DQB1*0602-positive subjects were sleepier and showed more fragmented sleep, despite SWE elevation comparable to negative subjects. Moreover, they showed differentially greater REM sleep latency reductions and smaller stage 2 reductions, along with differentially greater increases in fatigue. Both groups demonstrated comparable cumulative decreases in cognitive performance.
Conclusions: DQB1*0602 positivity in a healthy population may represent a continuum of some sleep-wake features of narcolepsy. DQB1*0602 was associated with interindividual differences in sleep homeostasis, physiologic sleep, sleepiness, and fatigue-but not in cognitive measures-during baseline and chronic PSD. Thus, DQB1*0602 may represent a genetic biomarker for predicting such individual differences in basal and sleep loss conditions.
Figures
Comment in
-
Why do we respond differently to sleep deprivation?: it's in our genes!Neurology. 2010 Oct 26;75(17):1492-3. doi: 10.1212/WNL.0b013e3181f9630d. Neurology. 2010. PMID: 20975049 No abstract available.
Similar articles
-
Faster REM sleep EEG and worse restedness in older insomniacs with HLA DQB1*0602.Psychiatry Res. 2011 May 30;187(3):397-400. doi: 10.1016/j.psychres.2011.01.007. Epub 2011 Feb 2. Psychiatry Res. 2011. PMID: 21292329 Free PMC article.
-
Catechol-O-methyltransferase Val158Met polymorphism associates with individual differences in sleep physiologic responses to chronic sleep loss.PLoS One. 2011;6(12):e29283. doi: 10.1371/journal.pone.0029283. Epub 2011 Dec 27. PLoS One. 2011. PMID: 22216231 Free PMC article.
-
Nocturnal sleep and daytime sleepiness in normal subjects with HLA-DQB1*0602.Sleep. 1999 May 1;22(3):347-52. Sleep. 1999. PMID: 10341385
-
Genetic studies in the sleep disorder narcolepsy.Genome Res. 1998 May;8(5):427-34. doi: 10.1101/gr.8.5.427. Genome Res. 1998. PMID: 9582188 Review.
-
Behavioral and genetic markers of sleepiness.J Clin Sleep Med. 2011 Oct 15;7(5 Suppl):S19-21. doi: 10.5664/JCSM.1348. J Clin Sleep Med. 2011. PMID: 22003324 Free PMC article. Review.
Cited by
-
Baseline sleep dysfunction among matriculating interns.J Grad Med Educ. 2012 Jun;4(2):202-8. doi: 10.4300/JGME-D-11-00153.1. J Grad Med Educ. 2012. PMID: 23730442 Free PMC article.
-
The BDNF Val66Met polymorphism modulates sleep intensity: EEG frequency- and state-specificity.Sleep. 2012 Mar 1;35(3):335-44. doi: 10.5665/sleep.1690. Sleep. 2012. PMID: 22379239 Free PMC article.
-
Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity.Sleep. 2015 Mar 1;38(3):351-60. doi: 10.5665/sleep.4488. Sleep. 2015. PMID: 25581913 Free PMC article.
-
Correlation of daytime sleepiness with urine metabolites in patients with obstructive sleep apnea.Sleep Breath. 2014 Sep;18(3):517-23. doi: 10.1007/s11325-013-0913-5. Epub 2014 Jan 9. Sleep Breath. 2014. PMID: 24402350
-
Sleepiness and Safety: Where Biology Needs Technology.Sleep Biol Rhythms. 2014 Apr 1;12(2):74-84. doi: 10.1111/sbr.12067. Sleep Biol Rhythms. 2014. PMID: 24955033 Free PMC article.
References
-
- Van Dongen HPA, Maislin G, Mullington JM, Dinges DF. The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep 2003;26:117–126. - PubMed
-
- Van Dongen HP, Baynard MD, Maislin G, Dinges DF. Systematic interindividual differences in neurobehavioral impairment from sleep loss: evidence of trait-like differential vulnerability. Sleep 2004;27:423–433. - PubMed
-
- Van Dongen HP, Maislin G, Dinges DF. Dealing with interindividual differences in the temporal dynamics of fatigue and performance: importance and techniques. Aviat Space Environ Med 2004;75:A147–154. - PubMed
-
- Van Dongen HPA, Dinges DF. Sleep, circadian rhythms, and psychomotor vigilance performance. Clin Sports Med 2005;24:237–249. - PubMed
-
- Leproult R, Colecchia EF, Berardi AM, Stickgold R, Kosslyn SM, Van Cauter E. Individual differences in subjective and objective alertness during sleep deprivation are stable and unrelated. Am J Physiol Regulat Integr Comp Physiol 2003;284:R280–R290. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
