Novel diagnostic biomarkers for prostate cancer
- PMID: 20975847
- PMCID: PMC2962426
- DOI: 10.7150/jca.1.150
Novel diagnostic biomarkers for prostate cancer
Abstract
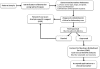
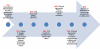
Prostate cancer is the most frequently diagnosed malignancy in American men, and a more aggressive form of the disease is particularly prevalent among African Americans. The therapeutic success rate for prostate cancer can be tremendously improved if the disease is diagnosed early. Thus, a successful therapy for this disease depends heavily on the clinical indicators (biomarkers) for early detection of the presence and progression of the disease, as well as the prediction after the clinical intervention. However, the current clinical biomarkers for prostate cancer are not ideal as there remains a lack of reliable biomarkers that can specifically distinguish between those patients who should be treated adequately to stop the aggressive form of the disease and those who should avoid overtreatment of the indolent form.A biomarker is a characteristic that is objectively measured and evaluated as an indicator of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention. A biomarker reveals further information to presently existing clinical and pathological analysis. It facilitates screening and detecting the cancer, monitoring the progression of the disease, and predicting the prognosis and survival after clinical intervention. A biomarker can also be used to evaluate the process of drug development, and, optimally, to improve the efficacy and safety of cancer treatment by enabling physicians to tailor treatment for individual patients. The form of the prostate cancer biomarkers can vary from metabolites and chemical products present in body fluid to genes and proteins in the prostate tissues.Current advances in molecular techniques have provided new tools facilitating the discovery of new biomarkers for prostate cancer. These emerging biomarkers will be beneficial and critical in developing new and clinically reliable indicators that will have a high specificity for the diagnosis and prognosis of prostate cancer. The purpose of this review is to examine the current status of prostate cancer biomarkers, with special emphasis on emerging markers, by evaluating their diagnostic and prognostic potentials. Both genes and proteins that reveal loss, mutation, or variation in expression between normal prostate and cancerous prostate tissues will be covered in this article. Along with the discovery of prostate cancer biomarkers, we will describe the criteria used when selecting potential biomarkers for further development towards clinical use. In addition, we will address how to appraise and validate candidate markers for prostate cancer and some relevant issues involved in these processes. We will also discuss the new concept of the biomarkers, existing challenges, and perspectives of biomarker development.
Keywords: diagnostic biomarkers; prostate cancer.
Conflict of interest statement
Conflict of Interest: The authors have declared that no conflict of interest exists.
Figures


References
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–49. - PubMed
-
- Home page of Prostate Cancer Foundation. Prostate Cancer Foundation; http://www.prostatecancerfoundation.org.
-
- Stanford JL, Stephenson RA, Coyle LM, Prostate Cancer Trends 1973-1995, NIH Pub No 99-4543. Bethesda, MD: SEER Program, National Cancer Institute; 1999.
-
- Prostate Cancer Screening: A Decision Guide for African Americans. US Department of Health and Human Services, Centers for Disease Control and Prevention (CDC); http://www.ustoo.org/PDFs/CDC_PCa_Screen_Guide_AA.pdf.
-
- Atkinson AJJr, Colburn WA, DeGruttola VG, DeMets DL. et al. Biomarkers and surrogate endpoints: Preferred definitions and conceptual framework. Biomarkers Definitions Working Group. Clinical Pharmacol & Therapeutics. 2001;69:89–95. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

