Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation
- PMID: 20975940
- PMCID: PMC2958807
- DOI: 10.1371/journal.pgen.1001165
Conjugative DNA transfer induces the bacterial SOS response and promotes antibiotic resistance development through integron activation
Abstract
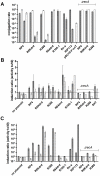
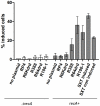
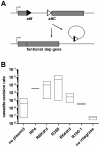
Conjugation is one mechanism for intra- and inter-species horizontal gene transfer among bacteria. Conjugative elements have been instrumental in many bacterial species to face the threat of antibiotics, by allowing them to evolve and adapt to these hostile conditions. Conjugative plasmids are transferred to plasmidless recipient cells as single-stranded DNA. We used lacZ and gfp fusions to address whether conjugation induces the SOS response and the integron integrase. The SOS response controls a series of genes responsible for DNA damage repair, which can lead to recombination and mutagenesis. In this manuscript, we show that conjugative transfer of ssDNA induces the bacterial SOS stress response, unless an anti-SOS factor is present to alleviate this response. We also show that integron integrases are up-regulated during this process, resulting in increased cassette rearrangements. Moreover, the data we obtained using broad and narrow host range plasmids strongly suggests that plasmid transfer, even abortive, can trigger chromosomal gene rearrangements and transcriptional switches in the recipient cell. Our results highlight the importance of environments concentrating disparate bacterial communities as reactors for extensive genetic adaptation of bacteria.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
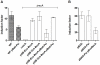

References
-
- Hammer BK, Bassler BL. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol Microbiol. 2003;50:101–104. - PubMed
-
- Barker MM, Gaal T, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. II. Models for positive control based on properties of RNAP mutants and competition for RNAP. J Mol Biol. 2001;305:689–702. - PubMed
-
- Barker MM, Gaal T, Josaitis CA, Gourse RL. Mechanism of regulation of transcription initiation by ppGpp. I. Effects of ppGpp on transcription initiation in vivo and in vitro. J Mol Biol. 2001;305:673–688. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

