Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication
- PMID: 20975956
- PMCID: PMC2958147
- DOI: 10.1371/journal.pone.0013521
Interferon-alpha mediates restriction of human immunodeficiency virus type-1 replication in primary human macrophages at an early stage of replication
Abstract
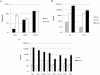
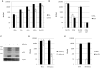
Type I interferons (IFNα and β) are induced directly in response to viral infection, resulting in an antiviral state for the cell. In vitro studies have shown that IFNα is a potent inhibitor of viral replication; however, its role in HIV-1 infection is incompletely understood. In this study we describe the ability of IFNα to restrict HIV-1 infection in primary human macrophages in contrast to peripheral blood mononuclear cells and monocyte-derived dendritic cells. Inhibition to HIV-1 replication in cells pretreated with IFNα occurred at an early stage in the virus life cycle. Late viral events such as budding and subsequent rounds of infection were not affected by IFNα treatment. Analysis of early and late HIV-1 reverse transcripts and integrated proviral DNA confirmed an early post entry role for IFNα. First strand cDNA synthesis was slightly reduced but late and integrated products were severely depleted, suggesting that initiation or the nucleic acid intermediates of reverse transcription are targeted. The depletion of integrated provirus is disproportionally greater than that of viral cDNA synthesis suggesting the possibility of a least an additional later target. A role for either cellular protein APOBEC3G or tetherin in this IFNα mediated restriction has been excluded. Vpu, previously shown by others to rescue a viral budding restriction by tetherin, could not overcome this IFNα induced effect. Determining both the viral determinants and cellular proteins involved may lead to novel therapeutic approaches. Our results add to the understanding of HIV-1 restriction by IFNα.
Conflict of interest statement
Figures


References
-
- Stark GR, Kerr IM, Williams BR, Silverman RH, Schreiber RD. How cells respond to interferons. Annu Rev Biochem. 1998;67:227–264. - PubMed
-
- Krown SE, Lee JY, Lin L, Fischl MA, Ambinder R, et al. Interferon-alpha2b with protease inhibitor-based antiretroviral therapy in patients with AIDS-associated Kaposi sarcoma: an AIDS malignancy consortium phase I trial. J Acquir Immune Defic Syndr. 2006;41:149–153. - PubMed
-
- Krown SE, Li P, Von Roenn JH, Paredes J, Huang J, et al. Efficacy of low-dose interferon with antiretroviral therapy in Kaposi's sarcoma: a randomized phase II AIDS clinical trials group study. J Interferon Cytokine Res. 2002;22:295–303. - PubMed
-
- Lane HC, Davey V, Kovacs JA, Feinberg J, Metcalf JA, et al. Interferon-alpha in patients with asymptomatic human immunodeficiency virus (HIV) infection. A randomized, placebo-controlled trial. Ann Intern Med. 1990;112:805–811. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

