The enantioselective intramolecular aminative functionalization of unactivated alkenes, dienes, allenes and alkynes for the synthesis of chiral nitrogen heterocycles
- PMID: 20976023
- PMCID: PMC2957828
- DOI: 10.1039/B907743J
The enantioselective intramolecular aminative functionalization of unactivated alkenes, dienes, allenes and alkynes for the synthesis of chiral nitrogen heterocycles
Abstract
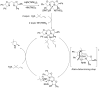
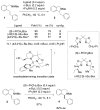
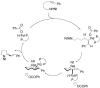
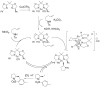
The enantioselective intramolecular aminative functionalization of unactivated alkenes and related π-systems is a straight-forward and atom economical strategy for the synthesis of chiral nitrogen heterocycles. These reactions can be categorized as oxidatively neutral, such as alkene hydroamination, or as oxidative reactions, such as alkene difunctionalization, e.g. aminooxygenation and carboamination. This perspective reviews the current work in the field and explores mechanistic trends that are common among the different catalysts and reaction types.
Figures


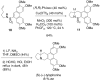
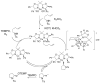
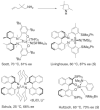
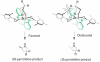
References
-
- Roesky PW, Muller TE. Angew. Chem., Int. Ed. 2003;42:2708–2710. - PubMed
-
- Hultzsch KC. Adv. Synth. Catal. 2005;347:367–391.
-
- Hultzsch KC. Org. Biomol. Chem. 2005;3:1819–1824. - PubMed
-
- Hong S, Marks TJ. Acc. Chem. Res. 2004;37:673–686. - PubMed
-
- Muller TE, Hultzsch KC, Yus M, Foubelo F, Tada M. Chem. Rev. 2008;108:3795–3892. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

