Information from pharmaceutical companies and the quality, quantity, and cost of physicians' prescribing: a systematic review
- PMID: 20976098
- PMCID: PMC2957394
- DOI: 10.1371/journal.pmed.1000352
Information from pharmaceutical companies and the quality, quantity, and cost of physicians' prescribing: a systematic review
Abstract
Background: Pharmaceutical companies spent $57.5 billion on pharmaceutical promotion in the United States in 2004. The industry claims that promotion provides scientific and educational information to physicians. While some evidence indicates that promotion may adversely influence prescribing, physicians hold a wide range of views about pharmaceutical promotion. The objective of this review is to examine the relationship between exposure to information from pharmaceutical companies and the quality, quantity, and cost of physicians' prescribing.
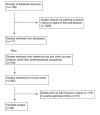
Methods and findings: We searched for studies of physicians with prescribing rights who were exposed to information from pharmaceutical companies (promotional or otherwise). Exposures included pharmaceutical sales representative visits, journal advertisements, attendance at pharmaceutical sponsored meetings, mailed information, prescribing software, and participation in sponsored clinical trials. The outcomes measured were quality, quantity, and cost of physicians' prescribing. We searched Medline (1966 to February 2008), International Pharmaceutical Abstracts (1970 to February 2008), Embase (1997 to February 2008), Current Contents (2001 to 2008), and Central (The Cochrane Library Issue 3, 2007) using the search terms developed with an expert librarian. Additionally, we reviewed reference lists and contacted experts and pharmaceutical companies for information. Randomized and observational studies evaluating information from pharmaceutical companies and measures of physicians' prescribing were independently appraised for methodological quality by two authors. Studies were excluded where insufficient study information precluded appraisal. The full text of 255 articles was retrieved from electronic databases (7,185 studies) and other sources (138 studies). Articles were then excluded because they did not fulfil inclusion criteria (179) or quality appraisal criteria (18), leaving 58 included studies with 87 distinct analyses. Data were extracted independently by two authors and a narrative synthesis performed following the MOOSE guidelines. Of the set of studies examining prescribing quality outcomes, five found associations between exposure to pharmaceutical company information and lower quality prescribing, four did not detect an association, and one found associations with lower and higher quality prescribing. 38 included studies found associations between exposure and higher frequency of prescribing and 13 did not detect an association. Five included studies found evidence for association with higher costs, four found no association, and one found an association with lower costs. The narrative synthesis finding of variable results was supported by a meta-analysis of studies of prescribing frequency that found significant heterogeneity. The observational nature of most included studies is the main limitation of this review.
Conclusions: With rare exceptions, studies of exposure to information provided directly by pharmaceutical companies have found associations with higher prescribing frequency, higher costs, or lower prescribing quality or have not found significant associations. We did not find evidence of net improvements in prescribing, but the available literature does not exclude the possibility that prescribing may sometimes be improved. Still, we recommend that practitioners follow the precautionary principle and thus avoid exposure to information from pharmaceutical companies. Please see later in the article for the Editors' Summary.
Conflict of interest statement
JL was paid for work with a Canadian generic company, Apotex Inc., in 2007. GS, PRM, BM, JL, NO, and AV are members of Healthy Skepticism Inc. Healthy Skepticism is an international nonprofit research, education, and advocacy association with the main aim of reducing harm from misleading health information. PRM is the director of Healthy Skepticism Inc., and is mostly unpaid. PRM's wife and two of his daughters are part-time employees of Healthy Skepticism Inc. JL is a member of the management committee of Healthy Skepticism.
Figures
Comment in
-
Doctors and drug companies: still cozy after all these years.PLoS Med. 2010 Nov 2;7(11):e1000359. doi: 10.1371/journal.pmed.1000359. PLoS Med. 2010. PMID: 21072244 Free PMC article.
References
-
- Gagnon MA, Lexchin J. The cost of pushing pills: a new estimate of pharmaceutical promotion expenditures in the United States. PLoS Med. 2008;5:e1. doi: 10.1371/journal.pmed.0050001. - DOI - PMC - PubMed
-
- Bras PL, Ricordeau P, Roussille B, Saintoyant V. L'information des médecins généralistes sur le médicament. Report No RM 2007-136 P. Inspection générale des affaires sociales. September 2007. Available: http://lesrapports.ladocumentationfrancaise.fr/BRP/074000703/0000.pdf. Accessed 10 July 2010.
-
- Garai P. Advertising and promotion of drugs. In: Talalay P, editor. Drugs in our society. Baltimore: John Hopkins Press; 1964.
-
- Pharmaceutical Research and Manufacturers of America (PhRMA) Code on Interactions with Healthcare Professionals. Available: http://www.phrma.org/code_on_interactions_with_healthcare_professionals. Accessed 8 July 2010.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources