Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy
- PMID: 20976099
- PMCID: PMC2957396
- DOI: 10.1371/journal.pbio.1000525
Filarial parasites develop faster and reproduce earlier in response to host immune effectors that determine filarial life expectancy
Abstract
Humans and other mammals mount vigorous immune assaults against helminth parasites, yet there are intriguing reports that the immune response can enhance rather than impair parasite development. It has been hypothesized that helminths, like many free-living organisms, should optimize their development and reproduction in response to cues predicting future life expectancy. However, immune-dependent development by helminth parasites has so far eluded such evolutionary explanation. By manipulating various arms of the immune response of experimental hosts, we show that filarial nematodes, the parasites responsible for debilitating diseases in humans like river blindness and elephantiasis, accelerate their development in response to the IL-5 driven eosinophilia they encounter when infecting a host. Consequently they produce microfilariae, their transmission stages, earlier and in greater numbers. Eosinophilia is a primary host determinant of filarial life expectancy, operating both at larval and at late adult stages in anatomically and temporally separate locations, and is implicated in vaccine-mediated protection. Filarial nematodes are therefore able to adjust their reproductive schedules in response to an environmental predictor of their probability of survival, as proposed by evolutionary theory, thereby mitigating the effects of the immune attack to which helminths are most susceptible. Enhancing protective immunity against filarial nematodes, for example through vaccination, may be less effective at reducing transmission than would be expected and may, at worst, lead to increased transmission and, hence, pathology.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

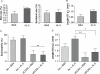
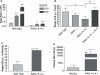
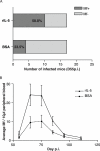
References
-
- Stearns S. C. The evolution of life histories. Oxford: Oxford University Press; 1992. 249
-
- Roff D. A. Life history evolution. Sunderland, Mass: Sinauer Associates; 2002. 527
-
- Weider L. J, Pijanowska J. Plasticity of Daphnia life histories in response to chemical cues from predators. Oikos. 1993;67:385–392.
-
- Stibor H, Luning J. Predator-induced phenotypic variation in the pattern of growth and reproduction in Daphnia hyalina (Crustacea: Cladocera). Funct Ecol. 1994;8:97–101.
-
- Bourdeau P. E. Cue reliability, risk sensitivity and inducible morphological defense in a marine snail. Oecologia. 2010;162:987–994. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

