Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila
- PMID: 20976106
- PMCID: PMC2956697
- DOI: 10.1371/journal.pone.0013477
Protein crosslinking by transglutaminase controls cuticle morphogenesis in Drosophila
Abstract
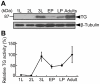
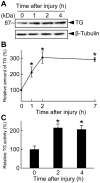
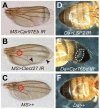
Transglutaminase (TG) plays important and diverse roles in mammals, such as blood coagulation and formation of the skin barrier, by catalyzing protein crosslinking. In invertebrates, TG is known to be involved in immobilization of invading pathogens at sites of injury. Here we demonstrate that Drosophila TG is an important enzyme for cuticle morphogenesis. Although TG activity was undetectable before the second instar larval stage, it dramatically increased in the third instar larval stage. RNA interference (RNAi) of the TG gene caused a pupal semi-lethal phenotype and abnormal morphology. Furthermore, TG-RNAi flies showed a significantly shorter life span than their counterparts, and approximately 90% of flies died within 30 days after eclosion. Stage-specific TG-RNAi before the third instar larval stage resulted in cuticle abnormality, but the TG-RNAi after the late pupal stage did not, indicating that TG plays a key role at or before the early pupal stage. Immediately following eclosion, acid-extractable protein from wild-type wings was nearly all converted to non-extractable protein due to wing maturation, whereas several proteins remained acid-extractable in the mature wings of TG-RNAi flies. We identified four proteins--two cuticular chitin-binding proteins, larval serum protein 2, and a putative C-type lectin-as TG substrates. RNAi of their corresponding genes caused a lethal phenotype or cuticle abnormality. Our results indicate that TG-dependent protein crosslinking in Drosophila plays a key role in cuticle morphogenesis and sclerotization.
Conflict of interest statement
Figures





Similar articles
-
Fondue and transglutaminase in the Drosophila larval clot.J Insect Physiol. 2008 Mar;54(3):586-92. doi: 10.1016/j.jinsphys.2007.12.008. Epub 2007 Dec 23. J Insect Physiol. 2008. PMID: 18222466
-
Transglutaminase-catalyzed protein-protein cross-linking suppresses the activity of the NF-κB-like transcription factor relish.Sci Signal. 2013 Jul 23;6(285):ra61. doi: 10.1126/scisignal.2003970. Sci Signal. 2013. PMID: 23882120
-
RNA Interference Directed against the Transglutaminase Gene Triggers Dysbiosis of Gut Microbiota in Drosophila.J Biol Chem. 2016 Nov 25;291(48):25077-25087. doi: 10.1074/jbc.M116.761791. Epub 2016 Oct 19. J Biol Chem. 2016. PMID: 27760824 Free PMC article.
-
Pluripotency and a secretion mechanism of Drosophila transglutaminase.J Biochem. 2018 Mar 1;163(3):165-176. doi: 10.1093/jb/mvx059. J Biochem. 2018. PMID: 28992227 Review.
-
Evolution of larval morphology in flies: get in shape with shavenbaby.Trends Genet. 2004 Jul;20(7):305-13. doi: 10.1016/j.tig.2004.05.003. Trends Genet. 2004. PMID: 15219395 Review. No abstract available.
Cited by
-
Single-cell profiling of the developing embryonic heart in Drosophila.Development. 2023 Aug 15;150(16):dev201936. doi: 10.1242/dev.201936. Epub 2023 Aug 24. Development. 2023. PMID: 37526610 Free PMC article.
-
Arthropod Innate Immune Systems and Vector-Borne Diseases.Biochemistry. 2017 Feb 21;56(7):907-918. doi: 10.1021/acs.biochem.6b00870. Epub 2017 Feb 8. Biochemistry. 2017. PMID: 28072517 Free PMC article. Review.
-
Properties of the cuticular proteins of Anopheles gambiae as revealed by serial extraction of adults.PLoS One. 2017 Apr 18;12(4):e0175423. doi: 10.1371/journal.pone.0175423. eCollection 2017. PLoS One. 2017. PMID: 28419115 Free PMC article.
-
Systemic coagulopathy promotes host lethality in a new Drosophila tumor model.Curr Biol. 2023 Jul 24;33(14):3002-3010.e6. doi: 10.1016/j.cub.2023.05.071. Epub 2023 Jun 23. Curr Biol. 2023. PMID: 37354901 Free PMC article.
-
Inhibition of transglutaminase exacerbates polyglutamine-induced neurotoxicity by increasing the aggregation of mutant ataxin-3 in an SCA3 Drosophila model.Neurotox Res. 2015 Apr;27(3):259-67. doi: 10.1007/s12640-014-9506-8. Epub 2014 Dec 11. Neurotox Res. 2015. PMID: 25501875
References
-
- Furie B, Furie BC. The molecular basis of blood coagulation. Cell. 1988;53:505–518. - PubMed
-
- Kalinin A, Marekov LN, Steinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114:3069–3070. - PubMed
-
- Lorand L, Graham RM. Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol. 2003;4:140–156. - PubMed
-
- Lorand L. Factor XIII: structure, activation, and interactions with fibrinogen and fibrin. Ann N Y Acad Sci. 2001;936:291–311. - PubMed
-
- Kopacek P, Hall M, Söderhäll K. Characterization of a clotting protein, isolated from plasma of the freshwater crayfish Pacifastacus leniusculus. Eur J Biochem. 1993;213:591–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

