Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells
- PMID: 20976141
- PMCID: PMC2956649
- DOI: 10.1371/journal.pone.0013428
Vitamin D binding protein-macrophage activating factor directly inhibits proliferation, migration, and uPAR expression of prostate cancer cells
Abstract
Background: Vitamin D binding protein-macrophage activating factor (DBP-maf) is a potent inhibitor of tumor growth. Its activity, however, has been attributed to indirect mechanisms such as boosting the immune response by activating macrophages and inhibiting the blood vessel growth necessary for the growth of tumors.
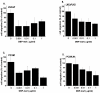
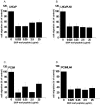
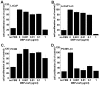
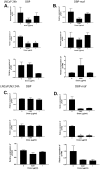
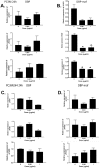
Methods and findings: In this study we show for the first time that DBP-maf exhibits a direct and potent effect on prostate tumor cells in the absence of macrophages. DBP-maf demonstrated inhibitory activity in proliferation studies of both LNCaP and PC3 prostate cancer cell lines as well as metastatic clones of these cells. Flow cytometry studies with annexin V and propidium iodide showed that this inhibitory activity is not due to apoptosis or cell death. DBP-maf also had the ability to inhibit migration of prostate cancer cells in vitro. Finally, DBP-maf was shown to cause a reduction in urokinase plasminogen activator receptor (uPAR) expression in prostate tumor cells. There is evidence that activation of this receptor correlates with tumor metastasis.
Conclusions: These studies show strong inhibitory activity of DBP-maf on prostate tumor cells independent of its macrophage activation.
Conflict of interest statement
Figures


References
-
- Kawakami M, Blum CB, Ramakrishnan R, Dell RB, Goodman DS. Turnover of the plasma binding protein for vitamin D and its metabolites in normal human subjects. J Clin Endocrinol Metab. 1981;53:1110–1116. - PubMed
-
- Yamamoto N, Naraparaju VR. Vitamin D3-binding protein as a precursor for macrophage activating factor in the inflammation-primed macrophage activation cascade in rats. Cell Immunol. 1996;170:161–167. - PubMed
-
- Kanda S, Mochizuki Y, Miyata Y, Kanetake H, Yamamoto N. Effects of vitamin D(3)-binding protein-derived macrophage activating factor (GcMAF) on angiogenesis. J Natl Cancer Inst. 2002;94:1311–1319. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

