The dopamine metabolite 3-methoxytyramine is a neuromodulator
- PMID: 20976142
- PMCID: PMC2956650
- DOI: 10.1371/journal.pone.0013452
The dopamine metabolite 3-methoxytyramine is a neuromodulator
Erratum in
- PLoS One. 2010;5(10) doi: 10.1371/annotation/a2019934-b1cc-4781-80cb-9e09fc7ff6dc.
Abstract
Dopamine (3-hydroxytyramine) is a well-known catecholamine neurotransmitter involved in multiple physiological functions including movement control. Here we report that the major extracellular metabolite of dopamine, 3-methoxytyramine (3-MT), can induce behavioral effects in a dopamine-independent manner and these effects are partially mediated by the trace amine associated receptor 1 (TAAR1). Unbiased in vivo screening of putative trace amine receptor ligands for potential effects on the movement control revealed that 3-MT infused in the brain is able to induce a complex set of abnormal involuntary movements in mice acutely depleted of dopamine. In normal mice, the central administration of 3-MT caused a temporary mild hyperactivity with a concomitant set of abnormal movements. Furthermore, 3-MT induced significant ERK and CREB phosphorylation in the mouse striatum, signaling events generally related to PKA-mediated cAMP accumulation. In mice lacking TAAR1, both behavioral and signaling effects of 3-MT were partially attenuated, consistent with the ability of 3-MT to activate TAAR1 receptors and cause cAMP accumulation as well as ERK and CREB phosphorylation in cellular assays. Thus, 3-MT is not just an inactive metabolite of DA, but a novel neuromodulator that in certain situations may be involved in movement control. Further characterization of the physiological functions mediated by 3-MT may advance understanding of the pathophysiology and pharmacology of brain disorders involving abnormal dopaminergic transmission, such as Parkinson's disease, dyskinesia and schizophrenia.
Conflict of interest statement
Figures
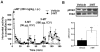
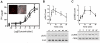
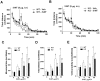

Similar articles
-
Modulation by Trace Amine-Associated Receptor 1 of Experimental Parkinsonism, L-DOPA Responsivity, and Glutamatergic Neurotransmission.J Neurosci. 2015 Oct 14;35(41):14057-69. doi: 10.1523/JNEUROSCI.1312-15.2015. J Neurosci. 2015. PMID: 26468205 Free PMC article.
-
Total glucosides of paeony (TGP) extracted from Radix Paeoniae Alba exerts neuroprotective effects in MPTP-induced experimental parkinsonism by regulating the cAMP/PKA/CREB signaling pathway.J Ethnopharmacol. 2019 Dec 5;245:112182. doi: 10.1016/j.jep.2019.112182. Epub 2019 Aug 22. J Ethnopharmacol. 2019. PMID: 31445131
-
G-protein coupled receptor 6 deficiency alters striatal dopamine and cAMP concentrations and reduces dyskinesia in a mouse model of Parkinson's disease.Exp Neurol. 2014 Jul;257:1-9. doi: 10.1016/j.expneurol.2014.04.010. Epub 2014 Apr 18. Exp Neurol. 2014. PMID: 24747358
-
Gα(olf) mutation allows parsing the role of cAMP-dependent and extracellular signal-regulated kinase-dependent signaling in L-3,4-dihydroxyphenylalanine-induced dyskinesia.J Neurosci. 2012 Apr 25;32(17):5900-10. doi: 10.1523/JNEUROSCI.0837-12.2012. J Neurosci. 2012. PMID: 22539851 Free PMC article.
-
Multiple treatments with L-3,4-dihydroxyphenylalanine modulate dopamine biosynthesis and neurotoxicity through the protein kinase A-transient extracellular signal-regulated kinase and exchange protein activation by cyclic AMP-sustained extracellular signal-regulated kinase signaling pathways.J Neurosci Res. 2014 Dec;92(12):1746-56. doi: 10.1002/jnr.23450. Epub 2014 Jul 12. J Neurosci Res. 2014. PMID: 25044243
Cited by
-
The Urinary Metabolome of Newborns with Perinatal Complications.Metabolites. 2024 Jan 10;14(1):41. doi: 10.3390/metabo14010041. Metabolites. 2024. PMID: 38248844 Free PMC article.
-
Multigenerational metabolic profiling in the Michigan PBB registry.Environ Res. 2019 May;172:182-193. doi: 10.1016/j.envres.2019.02.018. Epub 2019 Feb 13. Environ Res. 2019. PMID: 30782538 Free PMC article.
-
Trace amine-associated receptor 1 and drug abuse.Adv Pharmacol. 2022;93:373-401. doi: 10.1016/bs.apha.2021.10.005. Epub 2021 Nov 11. Adv Pharmacol. 2022. PMID: 35341572 Free PMC article.
-
Inhibition of PDE10A in a New Rat Model of Severe Dopamine Depletion Suggests New Approach to Non-Dopamine Parkinson's Disease Therapy.Biomolecules. 2022 Dec 21;13(1):9. doi: 10.3390/biom13010009. Biomolecules. 2022. PMID: 36671394 Free PMC article.
-
Cytotoxic, genotoxic, and neurotoxic effects of Mg, Pb, and Fe on pheochromocytoma (PC-12) cells.Environ Toxicol. 2015 Dec;30(12):1445-58. doi: 10.1002/tox.22014. Epub 2014 Jun 18. Environ Toxicol. 2015. PMID: 24942330 Free PMC article.
References
-
- Molinoff PB, Axelrod J. Biochemistry of catecholamines. Annu Rev Biochem. 1971;40:465–500. - PubMed
-
- Zhou QY, Palmiter RD. Dopamine-deficient mice are severely hypoactive, adipsic, and aphagic. Cell. 1995;83:1197–1209. - PubMed
-
- Carlsson A. Biochemical and pharmacological aspects of Parkinsonism. Acta Neurol Scand. 1972;51(Suppl):11–42. - PubMed
-
- Fon EA, Pothos EN, Sun BC, Killeen N, Sulzer D, et al. Vesicular transport regulates monoamine storage and release but is not essential for amphetamine action. Neuron. 1997;19:1271–1283. - PubMed
-
- Wang YM, Gainetdinov RR, Fumagalli F, Xu F, Jones SR, et al. Knockout of the vesicular monoamine transporter 2 gene results in neonatal death and supersensitivity to cocaine and amphetamine. Neuron. 1997;19:1285–1296. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

