Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs
- PMID: 20976144
- PMCID: PMC2956652
- DOI: 10.1371/journal.pone.0013453
Reversible block of mouse neural stem cell differentiation in the absence of dicer and microRNAs
Abstract
Background: To investigate the functions of Dicer and microRNAs in neural stem (NS) cell self-renewal and neurogenesis, we established neural stem cell lines from the embryonic mouse Dicer-null cerebral cortex, producing neural stem cell lines that lacked all microRNAs.
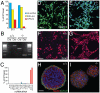
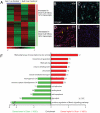
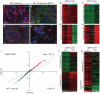
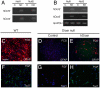
Principal findings: Dicer-null NS cells underwent normal self-renewal and could be maintained in vitro indefinitely, but had subtly altered cell cycle kinetics and abnormal heterochromatin organisation. In the absence of all microRNAs, Dicer-null NS cells were incapable of generating either glial or neuronal progeny and exhibited a marked dependency on exogenous EGF for survival. Dicer-null NS cells assumed complex differences in mRNA and protein expression under self-renewing conditions, upregulating transcripts indicative of self-renewing NS cells and expressing genes characteristic of differentiating neurons and glia. Underlining the growth-factor dependency of Dicer-null NS cells, many regulators of apoptosis were enriched in expression in these cells. Dicer-null NS cells initiate some of the same gene expression changes as wild-type cells under astrocyte differentiating conditions, but also show aberrant expression of large sets of genes and ultimately fail to complete the differentiation programme. Acute replacement of Dicer restored their ability to differentiate to both neurons and glia.
Conclusions: The block in differentiation due to loss of Dicer and microRNAs is reversible and the significantly altered phenotype of Dicer-null NS cells does not constitute a permanent transformation. We conclude that Dicer and microRNAs function in this system to maintain the neural stem cell phenotype and to facilitate the completion of differentiation.
Conflict of interest statement
Figures


References
-
- Bartel DP, Chen CZ. Micromanagers of gene expression: the potentially widespread influence of metazoan microRNAs. Nat Rev Genet. 2004;5:396–400. - PubMed
-
- Ambros V. The functions of animal microRNAs. Nature. 2004;431:350–355. - PubMed
-
- Kosik KS, Krichevsky AM. The Elegance of the MicroRNAs: A Neuronal Perspective. Neuron. 2005;47:779–782. - PubMed
-
- Cheng LC, Tavazoie M, Doetsch F. Stem cells: from epigenetics to microRNAs. Neuron. 2005;46:363–367. - PubMed
-
- Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, et al. Identification of tissue-specific microRNAs from mouse. Curr Biol. 2002;12:735–739. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

