Hyperglycemia induces oxidative stress and impairs axonal transport rates in mice
- PMID: 20976160
- PMCID: PMC2956689
- DOI: 10.1371/journal.pone.0013463
Hyperglycemia induces oxidative stress and impairs axonal transport rates in mice
Abstract
Background: While hyperglycemia-induced oxidative stress damages peripheral neurons, technical limitations have, in part, prevented in vivo studies to determine the effect of hyperglycemia on the neurons in the central nervous system (CNS). While olfactory dysfunction is indicated in diabetes, the effect of hyperglycemia on olfactory receptor neurons (ORNs) remains unknown. In this study, we utilized manganese enhanced MRI (MEMRI) to assess the impact of hyperglycemia on axonal transport rates in ORNs. We hypothesize that (i) hyperglycemia induces oxidative stress and is associated with reduced axonal transport rates in the ORNs and (ii) hyperglycemia-induced oxidative stress activates the p38 MAPK pathway in association with phosphorylation of tau protein leading to the axonal transport deficits.
Research design and methods: T(1)-weighted MEMRI imaging was used to determine axonal transport rates post-streptozotocin injection in wildtype (WT) and superoxide dismutase 2 (SOD2) overexpressing C57Bl/6 mice. SOD2 overexpression reduces mitochondrial superoxide load. Dihydroethidium staining was used to quantify the reactive oxygen species (ROS), specifically, superoxide (SO). Protein and gene expression levels were determined using western blotting and Q-PCR analysis, respectively.
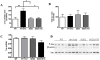
Results: STZ-treated WT mice exhibited significantly reduced axonal transport rates and significantly higher levels of ROS, phosphorylated p38 MAPK and tau protein as compared to the WT vehicle treated controls and STZ-treated SOD2 mice. The gene expression levels of p38 MAPK and tau remained unchanged.
Conclusion: Increased oxidative stress in STZ-treated WT hyperglycemic mice activates the p38 MAPK pathway in association with phosphorylation of tau and attenuates axonal transport rates in the olfactory system. In STZ-treated SOD-overexpressing hyperglycemic mice in which superoxide levels are reduced, these deficits are reversed.
Conflict of interest statement
Figures



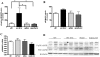
References
-
- Menne J, Park J-K, Boehne M, Elger M, Lindschau C, et al. Diminished loss of proteoglycans and lack of albuminuria in protein kinase C-alpha-deficient diabetic mice. Diabetes. 2004;53:2101–2109. - PubMed
-
- Dennis JC, Coleman ES, Swyers SE, Moody SW, Wright JC, et al. Changes in mitotic rate and GFAP expression in the primary olfactory axis of streptozotocin-induced diabetic rats. J Neurocytol. 2005;34:3–10. - PubMed
-
- Le Floch JP, Le Lievre G, Labroue M, Paul M, Peynegre R, et al. Smell dysfunction and related factors in diabetes. Diabetes Care. 1993;16:934–937. - PubMed
-
- Weinstock RS, Wright HN, Smith DU. Olfactory dysfunction in diabetes mellitus. Physiol Behav. 1993;53:17–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

