An efficient and versatile synthesis of GlcNAcstatins-potent and selective O-GlcNAcase inhibitors built on the tetrahydroimidazo[1,2-a]pyridine scaffold
- PMID: 20976183
- PMCID: PMC2956484
- DOI: 10.1016/j.tet.2010.07.037
An efficient and versatile synthesis of GlcNAcstatins-potent and selective O-GlcNAcase inhibitors built on the tetrahydroimidazo[1,2-a]pyridine scaffold
Abstract
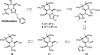
We report a novel approach to the synthesis of GlcNAcstatins-members of an emerging family of potent and selective inhibitors of peptidyl O-GlcNAc hydrolase build upon tetrahydroimidazo[1,2-a]pyridine scaffold. Making use of a streamlined synthetic sequence featuring de novo synthesis of imidazoles from glyoxal, ammonia and aldehydes, a properly functionalised linear GlcNAcstatin precursor has been efficiently prepared starting from methyl 3,4-O-(2',3'-dimethoxybutane-2',3'-diyl)-α-d-mannopyranoside. Subsequent ring closure of the linear precursor in an intramolecular S(N)2 process furnished the key fused d-mannose-imidazole GlcNAcstatin precursor in excellent yield. Finally, a sequence of transformations of this key intermediate granted expeditious access to a variety of the target compounds bearing a C(2)-phenethyl group and a range of N(8) acyl substituents. The versatility of the new approach stems from an appropriate choice of a set of acid labile permanent protecting groups on the monosaccharide starting material. Application was demonstrated by the synthesis of GlcNAcstatins containing polyunsaturated and thiol-containing amido substituents.
Figures
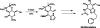
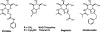




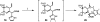

Similar articles
-
GlcNAcstatins are nanomolar inhibitors of human O-GlcNAcase inducing cellular hyper-O-GlcNAcylation.Biochem J. 2009 May 13;420(2):221-7. doi: 10.1042/BJ20090110. Biochem J. 2009. PMID: 19275764 Free PMC article.
-
Design, Synthesis, and Application of Chiral Bicyclic Imidazole Catalysts.Acc Chem Res. 2022 Sep 20;55(18):2708-2727. doi: 10.1021/acs.accounts.2c00455. Epub 2022 Aug 31. Acc Chem Res. 2022. PMID: 36043467
-
Enzyme molecular mechanism as a starting point to design new inhibitors: a theoretical study of O-GlcNAcase.J Phys Chem B. 2011 May 26;115(20):6764-75. doi: 10.1021/jp202079e. Epub 2011 May 4. J Phys Chem B. 2011. PMID: 21542586
-
Synthetic methodology utilized to prepare substituted imidazole p38 MAP kinase inhibitors.Curr Opin Drug Discov Devel. 2003 Nov;6(6):945-65. Curr Opin Drug Discov Devel. 2003. PMID: 14758763 Review.
-
Emerging roles of protein O-GlcNAcylation in cardiovascular diseases: Insights and novel therapeutic targets.Pharmacol Res. 2021 Mar;165:105467. doi: 10.1016/j.phrs.2021.105467. Epub 2021 Jan 27. Pharmacol Res. 2021. PMID: 33515704 Review.
Cited by
-
Protein O-GlcNAcylation in Cardiac Pathologies: Past, Present, Future.Front Endocrinol (Lausanne). 2019 Jan 15;9:819. doi: 10.3389/fendo.2018.00819. eCollection 2018. Front Endocrinol (Lausanne). 2019. PMID: 30697194 Free PMC article. Review.
-
O-GlcNAcase Fragment Discovery with Fluorescence Polarimetry.ACS Chem Biol. 2018 May 18;13(5):1353-1360. doi: 10.1021/acschembio.8b00183. Epub 2018 May 2. ACS Chem Biol. 2018. PMID: 29641181 Free PMC article.
References
-
- Torres C.R., Hart G.W. J. Biol. Chem. 1984;259:3308–3317. - PubMed
-
- Zachara N.E., Hart G.W. Biochim. Biophys. Acta. 2004;1673:13–28. - PubMed
-
- Love D.C., Hanover J.A. Sci. STKE. 2005;312:1–14. - PubMed
-
- Wells L., Gao Y., Mahoney J.A., Vosseller K., Chen C., Rosen A., Hart G.W. J. Biol. Chem. 2002;277:1755–1761. - PubMed
-
- Lehman D.M., Fu D.J., Freeman A.B., Hunt K.J., Leach R.J., Johnson-Pais T., HamLington J., Dyer T.D., Arya R., Abboud H., Goring H.H., Duggirala R., Blangero J., Konrad R.J., Stern M.P. Diabetes. 2005;54:1214–1221. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
