Diacylglycerol kinase β knockout mice exhibit lithium-sensitive behavioral abnormalities
- PMID: 20976192
- PMCID: PMC2956634
- DOI: 10.1371/journal.pone.0013447
Diacylglycerol kinase β knockout mice exhibit lithium-sensitive behavioral abnormalities
Abstract
Background: Diacylglycerol kinase (DGK) is an enzyme that phosphorylates diacylglycerol (DG) to produce phosphatidic acid (PA). DGKβ is widely distributed in the central nervous system, such as the olfactory bulb, cerebral cortex, striatum, and hippocampus. Recent studies reported that the splice variant at the COOH-terminal of DGKβ was related to bipolar disorder, but its detailed mechanism is still unknown.
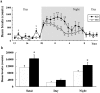
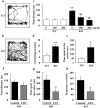
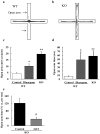
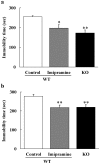
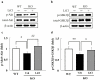
Methodology/principal findings: In the present study, we performed behavioral tests using DGKβ knockout (KO) mice to investigate the effects of DGKβ deficits on psychomotor behavior. DGKβ KO mice exhibited some behavioral abnormalities, such as hyperactivity, reduced anxiety, and reduced depression. Additionally, hyperactivity and reduced anxiety were attenuated by the administration of the mood stabilizer, lithium, but not haloperidol, diazepam, or imipramine. Moreover, DGKβ KO mice showed impairment in Akt-glycogen synthesis kinase (GSK) 3β signaling and cortical spine formation.
Conclusions/significance: These findings suggest that DGKβ KO mice exhibit lithium-sensitive behavioral abnormalities that are, at least in part, due to the impairment of Akt-GSK3β signaling and cortical spine formation.
Conflict of interest statement
Figures

Similar articles
-
Diacylglycerol kinase β knockout mice exhibit attention-deficit behavior and an abnormal response on methylphenidate-induced hyperactivity.PLoS One. 2012;7(5):e37058. doi: 10.1371/journal.pone.0037058. Epub 2012 May 10. PLoS One. 2012. PMID: 22590645 Free PMC article.
-
Deficiency of diacylglycerol kinase η induces lithium-sensitive mania-like behavior.J Neurochem. 2016 Aug;138(3):448-56. doi: 10.1111/jnc.13661. Epub 2016 May 30. J Neurochem. 2016. PMID: 27167678
-
Essential role of neuron-enriched diacylglycerol kinase (DGK), DGKbeta in neurite spine formation, contributing to cognitive function.PLoS One. 2010 Jul 15;5(7):e11602. doi: 10.1371/journal.pone.0011602. PLoS One. 2010. PMID: 20657643 Free PMC article.
-
Where do substrates of diacylglycerol kinases come from? Diacylglycerol kinases utilize diacylglycerol species supplied from phosphatidylinositol turnover-independent pathways.Adv Biol Regul. 2018 Jan;67:101-108. doi: 10.1016/j.jbior.2017.09.003. Epub 2017 Sep 9. Adv Biol Regul. 2018. PMID: 28918129 Review.
-
Diacylglycerol kinase β in neurons: functional implications at the synapse and in disease.Adv Biol Regul. 2012 May;52(2):315-25. doi: 10.1016/j.jbior.2012.03.003. Epub 2012 Mar 30. Adv Biol Regul. 2012. PMID: 22781745 Review.
Cited by
-
Alterations in acylcarnitines, amines, and lipids inform about the mechanism of action of citalopram/escitalopram in major depression.Transl Psychiatry. 2021 Mar 2;11(1):153. doi: 10.1038/s41398-020-01097-6. Transl Psychiatry. 2021. PMID: 33654056 Free PMC article.
-
Diacylglycerol Kinases as Emerging Potential Drug Targets for a Variety of Diseases: An Update.Front Cell Dev Biol. 2016 Aug 17;4:82. doi: 10.3389/fcell.2016.00082. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27583247 Free PMC article. Review.
-
Behavioral and pharmacological assessment of a potential new mouse model for mania.Physiol Behav. 2011 Jun 1;103(3-4):376-83. doi: 10.1016/j.physbeh.2011.03.005. Epub 2011 Mar 22. Physiol Behav. 2011. PMID: 21397618 Free PMC article.
-
Diacylglycerol kinase (DGKA) regulates the effect of the epilepsy and bipolar disorder treatment valproic acid in Dictyostelium discoideum.Dis Model Mech. 2018 Aug 21;11(9):dmm035600. doi: 10.1242/dmm.035600. Dis Model Mech. 2018. PMID: 30135067 Free PMC article.
-
Diacylglycerol kinases in membrane trafficking.Cell Logist. 2015 Aug 3;5(2):e1078431. doi: 10.1080/21592799.2015.1078431. eCollection 2015 Apr-Jun. Cell Logist. 2015. PMID: 27057419 Free PMC article. Review.
References
-
- Kanoh H, Yamada K, Sakane F. Diacylglycerol kinases: emerging downstream regulators in cell signaling systems. J Biochem. 2002;131:629–633. - PubMed
-
- Merida I, Avila-Flores A, Merino E. Diacylglycerol kinases: at the hub of cell signalling. Biochem J. 2008;409:1–18. - PubMed
-
- Topham MK, Prescott SM. Mammalian diacylglycerol kinases, a family of lipid kinases with signaling functions. J Biol Chem. 1999;274:11447–11450. - PubMed
-
- van Blitterswijk WJ, Houssa B. Properties and functions of diacylglycerol kinases. Cell Signal. 2000;12:595–605. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

