Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1
- PMID: 20976193
- PMCID: PMC2954834
- DOI: 10.1371/journal.ppat.1001144
Early production of IL-22 but not IL-17 by peripheral blood mononuclear cells exposed to live Borrelia burgdorferi: the role of monocytes and interleukin-1
Abstract
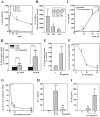
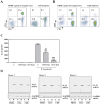
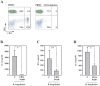
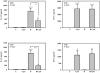
If insufficiently treated, Lyme borreliosis can evolve into an inflammatory disorder affecting skin, joints, and the CNS. Early innate immunity may determine host responses targeting infection. Thus, we sought to characterize the immediate cytokine storm associated with exposure of PBMC to moderate levels of live Borrelia burgdorferi. Since Th17 cytokines are connected to host defense against extracellular bacteria, we focused on interleukin (IL)-17 and IL-22. Here, we report that, despite induction of inflammatory cytokines including IL-23, IL-17 remained barely detectable in response to B. burgdorferi. In contrast, T cell-dependent expression of IL-22 became evident within 10 h of exposure to the spirochetes. This dichotomy was unrelated to interferon-γ but to a large part dependent on caspase-1 and IL-1 bioactivity derived from monocytes. In fact, IL-1β as a single stimulus induced IL-22 but not IL-17. Neutrophils display antibacterial activity against B. burgdorferi, particularly when opsonized by antibodies. Since neutrophilic inflammation, indicative of IL-17 bioactivity, is scarcely observed in Erythema migrans, a manifestation of skin inflammation after infection, protective and antibacterial properties of IL-22 may close this gap and serve essential functions in the initial phase of spirochete infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Aujla SJ, Kolls JK. IL-22: a critical mediator in mucosal host defense. J Mol Med. 2009;87:451–454. - PubMed
-
- Wolk K, Witte E, Witte K, Warszawska K, Sabat R. Biology of interleukin-22. Semin Immunopathol. 2010;32:17–31. - PubMed
-
- Miossec P, Korn T, Kuchroo VK. Interleukin-17 and type 17 helper T cells. N Engl J Med. 2009;361:888–898. - PubMed
-
- Steinman L. A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med. 2007;13:139–145. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

