Leishmania-induced inactivation of the macrophage transcription factor AP-1 is mediated by the parasite metalloprotease GP63
- PMID: 20976196
- PMCID: PMC2954837
- DOI: 10.1371/journal.ppat.1001148
Leishmania-induced inactivation of the macrophage transcription factor AP-1 is mediated by the parasite metalloprotease GP63
Abstract
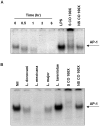
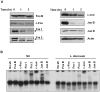
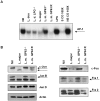
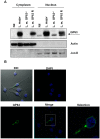
Leishmania parasites have evolved sophisticated mechanisms to subvert macrophage immune responses by altering the host cell signal transduction machinery, including inhibition of JAK/STAT signalling and other transcription factors such as AP-1, CREB and NF-κB. AP-1 regulates pro-inflammatory cytokines, chemokines and nitric oxide production. Herein we show that upon Leishmania infection, AP-1 activity within host cells is abolished and correlates with lower expression of 5 of the 7 AP-1 subunits. Of interest, c-Jun, the central component of AP-1, is cleaved by Leishmania. Furthermore, the cleavage of c-Jun is dependent on the expression and activity of the major Leishmania surface protease GP63. Immunoprecipitation of c-Jun from nuclear extracts showed that GP63 interacts, and cleaves c-Jun at the perinuclear area shortly after infection. Phagocytosis inhibition by cytochalasin D did not block c-Jun down-regulation, suggesting that internalization of the parasite might not be necessary to deliver GP63 molecules inside the host cell. This observation was corroborated by the maintenance of c-Jun cleavage upon incubation with L. mexicana culture supernatant, suggesting that secreted, soluble GP63 could use a phagocytosis-independent mechanism to enter the host cell. In support of this, disruption of macrophage lipid raft microdomains by Methyl β-Cyclodextrin (MβCD) partially inhibits the degradation of full length c-Jun. Together our results indicate a novel role of the surface protease GP63 in the Leishmania-mediated subversion of host AP-1 activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





References
-
- Desjeux P. Leishmaniasis: current situation and new perspectives. Comp Immunol Microbiol Infect Dis. 2004;27:305–318. - PubMed
-
- Forget G, Gregory DJ, Olivier M. Proteasome-mediated degradation of STAT1α following infection of macrophages with Leishmania donovani. J Biol Chem. 2005;280:30542–30549. - PubMed
-
- Gregory DJ, Contreras I, Forget G, Olivier M. A novel form of NF-κB is induced by Leishmania infection: Involvement in macrophage gene expression. Eur J Immunol. 2008;38:1071–1081. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

