Library of disordered patterns in 3D protein structures
- PMID: 20976197
- PMCID: PMC2954861
- DOI: 10.1371/journal.pcbi.1000958
Library of disordered patterns in 3D protein structures
Abstract
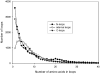
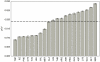
Intrinsically disordered regions serve as molecular recognition elements, which play an important role in the control of many cellular processes and signaling pathways. It is useful to be able to predict positions of disordered regions in protein chains. The statistical analysis of disordered residues was done considering 34,464 unique protein chains taken from the PDB database. In this database, 4.95% of residues are disordered (i.e. invisible in X-ray structures). The statistics were obtained separately for the N- and C-termini as well as for the central part of the protein chain. It has been shown that frequencies of occurrence of disordered residues of 20 types at the termini of protein chains differ from the ones in the middle part of the protein chain. Our systematic analysis of disordered regions in PDB revealed 109 disordered patterns of different lengths. Each of them has disordered occurrences in at least five protein chains with identity less than 20%. The vast majority of all occurrences of each disordered pattern are disordered. This allows one to use the library of disordered patterns for predicting the status of a residue of a given protein to be ordered or disordered. We analyzed the occurrence of the selected patterns in three eukaryotic and three bacterial proteomes.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- Tompa P. Intrinsically unstructured proteins. Trends Biochem Sci. 2002;27:527–533. - PubMed
-
- Wright PE, Dyson HJ. Intrinsically unstructured proteins: re-assessing the protein structure-function paradigm. J Mol Biol. 1999;293:321–331. - PubMed
-
- Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol. 2005;6:197–208. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

