Cost-effectiveness of intermittent preventive treatment of malaria in pregnancy in southern Mozambique
- PMID: 20976217
- PMCID: PMC2955525
- DOI: 10.1371/journal.pone.0013407
Cost-effectiveness of intermittent preventive treatment of malaria in pregnancy in southern Mozambique
Abstract
Background: Malaria in pregnancy is a public health problem for endemic countries. Economic evaluations of malaria preventive strategies in pregnancy are needed to guide health policies.
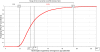
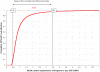
Methods and findings: This analysis was carried out in the context of a trial of malaria intermittent preventive treatment in pregnancy with sulphadoxine-pyrimethamine (IPTp-SP), where both intervention groups received an insecticide treated net through the antenatal clinic (ANC) in Mozambique. The cost-effectiveness of IPTp-SP on maternal clinical malaria and neonatal survival was estimated. Correlation and threshold analyses were undertaken to assess the main factors affecting the economic outcomes and the cut-off values beyond which the intervention is no longer cost-effective. In 2007 US$, the incremental cost-effectiveness ratio (ICER) for maternal malaria was 41.46 US$ (95% CI 20.5, 96.7) per disability-adjusted life-year (DALY) averted. The ICER per DALY averted due to the reduction in neonatal mortality was 1.08 US$ (95% CI 0.43, 3.48). The ICER including both the effect on the mother and on the newborn was 1.02 US$ (95% CI 0.42, 3.21) per DALY averted. Efficacy was the main factor affecting the economic evaluation of IPTp-SP. The intervention remained cost-effective with an increase in drug cost per dose up to 11 times in the case of maternal malaria and 183 times in the case of neonatal mortality.
Conclusions: IPTp-SP was highly cost-effective for both prevention of maternal malaria and reduction of neonatal mortality in Mozambique. These findings are likely to hold for other settings where IPTp-SP is implemented through ANC visits. The intervention remained cost-effective even with a significant increase in drug and other intervention costs. Improvements in the protective efficacy of the intervention would increase its cost-effectiveness. Provision of IPTp with a more effective, although more expensive drug than SP may still remain a cost-effective public health measure to prevent malaria in pregnancy.
Trial registration: ClinicalTrials.gov NCT00209781.
Conflict of interest statement
Figures


References
-
- Menéndez C, Ordi J, Ismail MR, Ventura PJ, Aponte JJ, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis. 2000;181:1740–1745. - PubMed
-
- Desai M, ter Kuile FO, Nosten F, McGready R, Asamoa K, et al. Epidemiology and burden of malaria in pregnancy. Lancet Infect Dis. 2007;7:93–104. - PubMed
-
- WHO . Geneva: World Health Organization; 2004. A strategic framework for malaria prevention and control during pregnancy in the African region.
-
- WHO . Geneva: World Health Organization; 2000. WHO Expert Committee on Malaria. - PubMed
-
- Mbonye AK, Hansen KS, Bygbjerg IC, Magnussen P. Intermittent preventive treatment of malaria in pregnancy: the incremental cost-effectiveness of a new delivery system in Uganda. Trans R Soc Trop Med Hyg. 2008;102:685–693. - PubMed

