4-oxo-N-(4-hydroxyphenyl)retinamide: two independent ways to kill cancer cells
- PMID: 20976277
- PMCID: PMC2954786
- DOI: 10.1371/journal.pone.0013362
4-oxo-N-(4-hydroxyphenyl)retinamide: two independent ways to kill cancer cells
Abstract
Background: The retinoid 4-oxo-N-(4-hydroxyphenyl)retinamide (4-oxo-4-HPR) is a polar metabolite of fenretinide (4-HPR) very effective in killing cancer cells of different histotypes, able to inhibit 4-HPR-resistant cell growth and to act synergistically in combination with the parent drug. Unlike 4-HPR and other retinoids, 4-oxo-4-HPR inhibits tubulin polymerization, leading to multipolar spindle formation and mitotic arrest. Here we investigated whether 4-oxo-4-HPR, like 4-HPR, triggered cell death also via reactive oxygen species (ROS) generation and whether its antimicrotubule activity was related to a ROS-dependent mechanism in ovarian (A2780), breast (T47D), cervical (HeLa) and neuroblastoma (SK-N-BE) cancer cell lines.
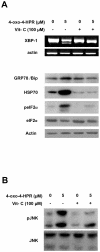
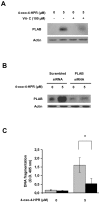
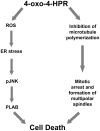
Methodology/principal findings: We provided evidence that 4-oxo-4-HPR, besides acting as an antimicrotubule agent, induced apoptosis through a signaling cascade starting from ROS generation and involving endoplasmic reticulum (ER) stress response, Jun N-terminal Kinase (JNK) activation, and upregulation of the proapoptotic PLAcental Bone morphogenetic protein (PLAB). Through time-course analysis and inhibition of the ROS-related signaling pathway (upstream by vitamin C and downstream by PLAB silencing), we demonstrated that the antimitotic activity of 4-oxo-4-HPR was independent from the oxidative stress induced by the retinoid. In fact, ROS generation occurred earlier than mitotic arrest (within 30 minutes and 2 hours, respectively) and abrogation of the ROS-related signaling pathway did not prevent the 4-oxo-4-HPR-induced mitotic arrest.
Conclusions/significance: These data indicate that 4-oxo-4-HPR anticancer activity is due to at least two independent mechanisms and provide an explanation of the ability of 4-oxo-4-HPR to be more potent than the parent drug and to be effective also in 4-HPR-resistant cell lines. In addition, the double mechanism of action could allow 4-oxo-4-HPR to efficiently target tumour and to eventually counteract the development of drug resistance.
Conflict of interest statement
Figures




Similar articles
-
AF1q: a novel mediator of basal and 4-HPR-induced apoptosis in ovarian cancer cells.PLoS One. 2012;7(6):e39968. doi: 10.1371/journal.pone.0039968. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761939 Free PMC article.
-
4-oxo-fenretinide, a recently identified fenretinide metabolite, induces marked G2-M cell cycle arrest and apoptosis in fenretinide-sensitive and fenretinide-resistant cell lines.Cancer Res. 2006 Mar 15;66(6):3238-47. doi: 10.1158/0008-5472.CAN-05-3362. Cancer Res. 2006. PMID: 16540676
-
Antimitotic effect of the retinoid 4-oxo-fenretinide through inhibition of tubulin polymerization: a novel mechanism of retinoid growth-inhibitory activity.Mol Cancer Ther. 2009 Dec;8(12):3360-8. doi: 10.1158/1535-7163.MCT-09-0798. Mol Cancer Ther. 2009. PMID: 19996280
-
Molecular mechanisms of fenretinide-induced apoptosis of neuroblastoma cells.Ann N Y Acad Sci. 2004 Dec;1028:81-9. doi: 10.1196/annals.1322.009. Ann N Y Acad Sci. 2004. PMID: 15650234 Review.
-
Clinical development of fenretinide as an antineoplastic drug: Pharmacology perspectives.Exp Biol Med (Maywood). 2017 Jun;242(11):1178-1184. doi: 10.1177/1535370217706952. Epub 2017 Apr 21. Exp Biol Med (Maywood). 2017. PMID: 28429653 Free PMC article. Review.
Cited by
-
Fenretinide Perturbs Focal Adhesion Kinase in Premalignant and Malignant Human Oral Keratinocytes. Fenretinide's Chemopreventive Mechanisms Include ECM Interactions.Cancer Prev Res (Phila). 2015 May;8(5):419-30. doi: 10.1158/1940-6207.CAPR-14-0418. Epub 2015 Feb 24. Cancer Prev Res (Phila). 2015. PMID: 25712051 Free PMC article.
-
Sodium 4-Carboxymethoxyimino-(4-HPR) a Novel Water-Soluble Derivative of 4-Oxo-4-HPR Endowed with In Vivo Anticancer Activity on Solid Tumors.Front Pharmacol. 2017 Apr 26;8:226. doi: 10.3389/fphar.2017.00226. eCollection 2017. Front Pharmacol. 2017. PMID: 28491037 Free PMC article.
-
AF1q: a novel mediator of basal and 4-HPR-induced apoptosis in ovarian cancer cells.PLoS One. 2012;7(6):e39968. doi: 10.1371/journal.pone.0039968. Epub 2012 Jun 26. PLoS One. 2012. PMID: 22761939 Free PMC article.
-
Inhibitory effects of fenretinide metabolites N-[4-methoxyphenyl]retinamide (MPR) and 4-oxo-N-(4-hydroxyphenyl)retinamide (3-keto-HPR) on fenretinide molecular targets β-carotene oxygenase 1, stearoyl-CoA desaturase 1 and dihydroceramide Δ4-desaturase 1.PLoS One. 2017 Apr 27;12(4):e0176487. doi: 10.1371/journal.pone.0176487. eCollection 2017. PLoS One. 2017. PMID: 28448568 Free PMC article.
References
-
- Nagy L, Thomazy VA, Heyman RA, Davies PJ. Retinoid-induced apoptosis in normal and neoplastic tissues. Cell Death Differ. 1998;5:11–19. - PubMed
-
- Veronesi U, De Palo G, Marubini E, Costa A, Formelli F, et al. Randomized trial of fenretinide to prevent second breast malignancy in women with early breast cancer. J Natl Cancer Inst. 1999;91:1847–1856. - PubMed
-
- Chiesa F, Tradati N, Grigolato R, Boracchi P, Biganzoli E, et al. Randomized trial of fenretinide (4HPR) to prevent recurrences, new localizations and carcinomas in patients operated on for oral leukoplakia: long-term results. Int J Cancer. 2005;115:625–629. - PubMed
-
- Moglia D, Formelli F, Baliva G, Bono A, Accetturi M, et al. Effects of topical treatment with fenretinide (4-HPR) and plasma vitamin A levels in patients with actinic keratoses. Cancer Lett. 1996;110:87–91. - PubMed
-
- Tradati N, Chiesa F, Rossi N, Grigolato R, Formelli F, et al. Successful topical treatment of oral lichen planus and leukoplakias with fenretinide (4-HPR). Cancer Lett. 1994;76:109–111. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

