p21 loss blocks senescence following Apc loss and provokes tumourigenesis in the renal but not the intestinal epithelium
- PMID: 20976827
- PMCID: PMC3394507
- DOI: 10.1002/emmm.201000101
p21 loss blocks senescence following Apc loss and provokes tumourigenesis in the renal but not the intestinal epithelium
Abstract
Senescence has been implicated as an important mechanism of tumour suppression in a number of human malignancies, including colorectal cancer (CRC). However, we still have a relatively poor understanding of how the underlying mutations that occur in cancer cause senescence and its relevance in vivo. The Apc gene is mutated in approximately 80% of CRC as the initiating event, but rarely elsewhere. In this study we have examined the capacity of Apc loss to induce senescence in the intestinal epithelium compared to the renal epithelium. Within the renal epithelium, loss of Apc function led to an induction of senescence, however, bypassing senescence through combined Apc and p21 or Ink4A gene deletion rapidly initiated renal carcinoma. Within the intestinal epithelium, loss of Apc did not induce senescence. Moreover, combined Apc and p21 or Ink4A loss had no impact upon tumourigenesis. Taken together, these results show that Apc loss in vivo invokes a senescence program in a context-dependent fashion, and implies senescence may play a key barrier to tumourigenesis in the kidney. However, in CRC, escape from senescence is likely to only be a barrier in cancers initiated by other mutations.
Figures


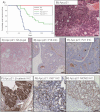



Kaplan–Meier survival graph showing no acceleration of intestinal tumourigenesis between AhCre+ Apcfl/+ (Apcfl/+, n = 15, black line) and AhCre+ Apcfl/+ p21−/− (Apcfl/+ p21−/−, n = 24, red line) mice (Log rank, p = 0.476). No significant differences were observed either in total tumour number (Mann–Whitney, p = 0.1893) (B), or average tumour area (Mann–Whitney, p = 0.1346) (C).
Survival graph showing no acceleration of tumourigenesis between Min (ApcMin/+, n = 20 black line), Min Ink4a+/− (ApcMin/+ Ink4a+/−, n = 23, red line) and Min Ink4a−/− (ApcMin/+ Ink4a−/−, n = 25, green line) mice (Log rank, p = 0.825). No significant differences were observed either in total tumour number (Mann–Whitney p = 0.7405) (E) or average tumour size (Mann–Whitney, p = 0.1601) (F).

Similar articles
-
Proteomic profiling of a mouse model of acute intestinal Apc deletion leads to identification of potential novel biomarkers of human colorectal cancer (CRC).Biochem Biophys Res Commun. 2013 Oct 25;440(3):364-70. doi: 10.1016/j.bbrc.2013.08.076. Epub 2013 Aug 31. Biochem Biophys Res Commun. 2013. PMID: 23998936
-
Global increase of p16INK4a in APC-deficient mouse liver drives clonal growth of p16INK4a-negative tumors.Mol Cancer Res. 2015 Feb;13(2):239-49. doi: 10.1158/1541-7786.MCR-14-0278-T. Epub 2014 Sep 30. Mol Cancer Res. 2015. PMID: 25270420
-
Loss of APC induces polyploidy as a result of a combination of defects in mitosis and apoptosis.J Cell Biol. 2007 Jan 15;176(2):183-95. doi: 10.1083/jcb.200610099. J Cell Biol. 2007. PMID: 17227893 Free PMC article.
-
Tissue-specific tumour suppression by APC.Adv Exp Med Biol. 2009;656:107-18. Adv Exp Med Biol. 2009. PMID: 19928356 Review.
-
Adenomatous polyposis coli (APC) plays multiple roles in the intestinal and colorectal epithelia.Med Mol Morphol. 2007 Jun;40(2):68-81. doi: 10.1007/s00795-006-0352-5. Epub 2007 Jun 18. Med Mol Morphol. 2007. PMID: 17572842 Review.
Cited by
-
After Nf1 loss in Schwann cells, inflammation drives neurofibroma formation.Neurooncol Adv. 2019 Nov 22;2(Suppl 1):i23-i32. doi: 10.1093/noajnl/vdz045. eCollection 2020 Jul. Neurooncol Adv. 2019. PMID: 32642730 Free PMC article. Review.
-
Cilia and cilia-associated proteins in cancer.Drug Discov Today Dis Mech. 2013 Dec 1;10(3-4):e135-e142. doi: 10.1016/j.ddmec.2013.03.004. Drug Discov Today Dis Mech. 2013. PMID: 24982684 Free PMC article.
-
Mice with renal-specific alterations of stem cell-associated signaling develop symptoms of chronic kidney disease but surprisingly no tumors.PLoS One. 2024 Mar 21;19(3):e0282938. doi: 10.1371/journal.pone.0282938. eCollection 2024. PLoS One. 2024. PMID: 38512983 Free PMC article.
-
Cellular senescence in renal ageing and disease.Nat Rev Nephrol. 2017 Feb;13(2):77-89. doi: 10.1038/nrneph.2016.183. Epub 2016 Dec 28. Nat Rev Nephrol. 2017. PMID: 28029153 Review.
-
Sleeping Beauty transposon mutagenesis in mouse intestinal organoids identifies genes involved in tumor progression and metastasis.Cancer Gene Ther. 2024 Apr;31(4):527-536. doi: 10.1038/s41417-023-00723-x. Epub 2024 Jan 4. Cancer Gene Ther. 2024. PMID: 38177308
References
-
- Andreu P, Colnot S, Godard C, Gad S, Chafey P, Niwa-Kawakita M, Laurent-Puig P, Kahn A, Robine S, Perret C, et al. Crypt-restricted proliferation and commitment to the Paneth cell lineage following Apc loss in the mouse intestine. Development. 2005;132:1443–1451. - PubMed
-
- Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, Haegebarth A, Korving J, Begthel H, Peters PJ, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, Danenberg E, Clarke AR, Sansom OJ, Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–611. - PubMed
-
- Bartek J, Lukas J, Bartkova J. DNA damage response as an anti-cancer barrier—damage threshold and the concept of ‘conditional haploinsufficiency’. Cell Cycle. 2007;6:2344–2347. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

