Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery
- PMID: 20977190
- PMCID: PMC3040098
- DOI: 10.1021/mp100203a
Modulation of CD4+ T lymphocyte lineage outcomes with targeted, nanoparticle-mediated cytokine delivery
Abstract
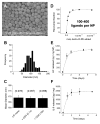
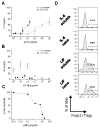
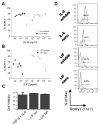
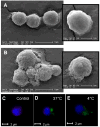
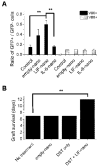
Within the immune system there is an exquisite ability to discriminate between "self" and "non-self" that is orchestrated by antigen-specific T lymphocytes. Genomic plasticity enables differentiation of naive CD4+ T lymphocytes into either regulatory cells (Treg) that express the transcription factor Foxp3 and actively prevent autoimmune self-destruction or effector cells (Teff) that attack and destroy their cognate target. An example of such plasticity is our recent discovery that leukemia inhibitory factor (LIF) supports Treg maturation in contrast to IL-6, which drives development of the pathogenic Th17 effector phenotype. This has revealed a LIF/IL6 axis in T cell development which can be exploited for modulation using targeted cytokine delivery. Here we demonstrate that LIF-loaded nanoparticles (NPs) directed to CD4+ T cells (i) oppose IL6-driven Th17 development; (ii) prolong survival of vascularized heart grafts in mice; and (iii) expand FOXP3+ CD4+ T cell numbers in a non-human primate model in vitro. In contrast, IL-6 loaded nanoparticles directed to CD4+ T cells increase Th17 development. Notably, nanoparticle-mediated delivery was demonstrated to be critical: unloaded nanoparticles and soluble LIF or IL-6 controls failed to recapitulate the efficacy of cytokine-loaded nanoparticles in induction and/or expansion of Foxp3+ cells or Th17 cells. Thus, this targeted nanoparticle approach is able to harness endogenous immune-regulatory pathways, providing a powerful new method to modulating T cell developmental plasticity in immune-mediated disease indications.
Conflict of interest statement
The authors declare no competing financial interests.
Figures


References
-
- Masteller EL, Tang Q, Bluestone JA. Antigen-specific regulatory T cells--ex vivo expansion and therapeutic potential. Semin Immunol. 2006;18(2):103–10. - PubMed
-
- Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–55. - PubMed
-
- Littman DR, Rudensky AY. Th17 and regulatory T cells in mediating and restraining inflammation. Cell. 140(6):845–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

