Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis
- PMID: 20977351
- PMCID: PMC3110094
- DOI: 10.1089/ars.2010.3682
Peroxide stress elicits adaptive changes in bacterial metal ion homeostasis
Abstract
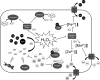
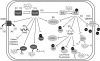
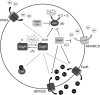
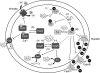
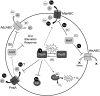
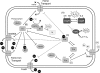
Exposure to hydrogen peroxide (H(2)O(2)) and other reactive oxygen species is a universal feature of life in an aerobic environment. Bacteria express enzymes to detoxify H(2)O(2) and to repair the resulting damage, and their synthesis is typically regulated by redox-sensing transcription factors. The best characterized bacterial peroxide-sensors are Escherichia coli OxyR and Bacillus subtilis PerR. Analysis of their regulons has revealed that, in addition to inducible detoxification enzymes, adaptation to H(2)O(2) is mediated by modifications of metal ion homeostasis. Analogous adaptations appear to be present in other bacteria as here reviewed for Deinococcus radiodurans, Neisseria gonorrhoeae, Streptococcus pyogenes, and Bradyrhizobium japonicum. As a general theme, peroxide stress elicits changes in cytosolic metal distribution with the net effect of reducing the damage caused by reactive ferrous iron. Iron levels are reduced by repression of uptake, sequestration in storage proteins, and incorporation into metalloenzymes. In addition, peroxide-inducible transporters elevate cytosolic levels of Mn(II) and/or Zn(II) that can displace ferrous iron from sensitive targets. Although bacteria differ significantly in the detailed mechanisms employed to modulate cytosolic metal levels, a high Mn:Fe ratio has emerged as one key correlate of reactive oxygen species resistance.
Figures

References
-
- Altuvia S. Almiron M. Huisman G. Kolter R. Storz G. The dps promoter is activated by OxyR during growth and by IHF and sigma S in stationary phase. Mol Microbiol. 1994;13:265–272. - PubMed
-
- Andrews SC. The Ferritin-like superfamily: evolution of the biological iron storeman from a rubrerythrin-like ancestor. Biochim Biophys Acta. 2010;1800:691–705. - PubMed
-
- Andrews SC. Robinson AK. Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev. 2003;27:215–237. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

