Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase
- PMID: 20977462
- PMCID: PMC2998693
- DOI: 10.1111/j.1476-5381.2010.00956.x
Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase
Abstract
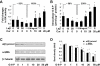
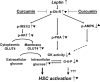
Background and purpose: Hyperleptinemia is commonly found in obese patients, associated with non-alcoholic steatohepatitis and hepatic fibrosis. Hepatic stellate cells (HSCs) are the most relevant effectors during hepatic fibrogenesis. We recently reported that leptin stimulated HSC activation, which was eliminated by curcumin, a phytochemical from turmeric. This study was designed to explore the underlying mechanisms, focusing on their effects on intracellular glucose in HSCs. We hypothesized that leptin stimulated HSC activation by elevating the level of intracellular glucose, which was eliminated by curcumin by inhibiting the membrane translocation of glucose transporter-4 (GLUT4) and inducing the conversion of glucose to glucose-6-phosphate (G-6-P).
Experimental approach: Levels of intracellular glucose were measured in rat HSCs and immortalized human hepatocytes. Contents of GLUT4 in cell fractions were analysed by Western blotting analyses. Activation of signalling pathways was assessed by comparing phosphorylation levels of protein kinases.
Key results: Leptin elevated the level of intracellular glucose in cultured HSCs, which was diminished by curcumin. Curcumin suppressed the leptin-induced membrane translocation of GLUT4 by interrupting the insulin receptor substrates/phosphatidyl inositol 3-kinase/AKT signalling pathway. Furthermore, curcumin stimulated glucokinase activity, increasing conversion of glucose to G-6-P.
Conclusions and implications: Curcumin prevented leptin from elevating levels of intracellular glucose in activated HSCs in vitro by inhibiting the membrane translocation of GLUT4 and stimulating glucose conversion, leading to the inhibition of HSC activation. Our results provide novel insights into mechanisms of curcumin in inhibiting leptin-induced HSC activation.
© 2010 The Authors. British Journal of Pharmacology © 2010 The British Pharmacological Society.
Figures





Similar articles
-
Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling.Lab Invest. 2014 May;94(5):503-16. doi: 10.1038/labinvest.2014.42. Epub 2014 Mar 10. Lab Invest. 2014. PMID: 24614199 Free PMC article.
-
Curcumin protects hepatic stellate cells against leptin-induced activation in vitro by accumulating intracellular lipids.Endocrinology. 2010 Sep;151(9):4168-77. doi: 10.1210/en.2010-0191. Epub 2010 Jul 21. Endocrinology. 2010. PMID: 20660066 Free PMC article.
-
Curcumin diminishes the impacts of hyperglycemia on the activation of hepatic stellate cells by suppressing membrane translocation and gene expression of glucose transporter-2.Mol Cell Endocrinol. 2011 Feb 20;333(2):160-71. doi: 10.1016/j.mce.2010.12.028. Epub 2010 Dec 30. Mol Cell Endocrinol. 2011. PMID: 21195127 Free PMC article.
-
Curcumin eliminates leptin's effects on hepatic stellate cell activation via interrupting leptin signaling.Endocrinology. 2009 Jul;150(7):3011-20. doi: 10.1210/en.2008-1601. Epub 2009 Mar 19. Endocrinology. 2009. PMID: 19299451 Free PMC article.
-
Curcumin targets multiple pathways to halt hepatic stellate cell activation: updated mechanisms in vitro and in vivo.Dig Dis Sci. 2015 Jun;60(6):1554-64. doi: 10.1007/s10620-014-3487-6. Epub 2014 Dec 23. Dig Dis Sci. 2015. PMID: 25532502 Review.
Cited by
-
Curcumin ameliorates hepatic fibrosis in type 2 diabetes mellitus - insights into its mechanisms of action.Br J Pharmacol. 2012 Aug;166(8):2209-11. doi: 10.1111/j.1476-5381.2012.01959.x. Br J Pharmacol. 2012. PMID: 22452372 Free PMC article.
-
Effects of curcumin on ion channels and transporters.Front Physiol. 2014 Mar 11;5:94. doi: 10.3389/fphys.2014.00094. eCollection 2014. Front Physiol. 2014. PMID: 24653706 Free PMC article. Review.
-
Purinergic receptor X7 mediates leptin induced GLUT4 function in stellate cells in nonalcoholic steatohepatitis.Biochim Biophys Acta. 2016 Jan;1862(1):32-45. doi: 10.1016/j.bbadis.2015.10.009. Epub 2015 Oct 22. Biochim Biophys Acta. 2016. PMID: 26474534 Free PMC article.
-
Curcumin eliminates the effect of advanced glycation end-products (AGEs) on the divergent regulation of gene expression of receptors of AGEs by interrupting leptin signaling.Lab Invest. 2014 May;94(5):503-16. doi: 10.1038/labinvest.2014.42. Epub 2014 Mar 10. Lab Invest. 2014. PMID: 24614199 Free PMC article.
-
Overview of Curcumin and Piperine Effects on Glucose Metabolism: The Case of an Insulinoma Patient's Loss of Consciousness.Int J Mol Sci. 2023 Apr 1;24(7):6621. doi: 10.3390/ijms24076621. Int J Mol Sci. 2023. PMID: 37047589 Free PMC article. Review.
References
-
- Aleffi S, Petrai I, Bertolani C, Parola M, Colombatto S, Novo E, et al. Upregulation of proinflammatory and proangiogenic cytokines by leptin in human hepatic stellate cells. Hepatology. 2005;42:1339–1348. - PubMed
-
- Asano T, Ogihara T, Katagiri H, Sakoda H, Ono H, Fujishiro M, et al. Glucose transporter and Na+/glucose cotransporter as molecular targets of anti-diabetic drugs. Curr Med Chem. 2004;11:2717–2724. - PubMed
-
- Bae JH, Park JW, Kwon TK. Ruthenium red, inhibitor of mitochondrial Ca2+ uniporter, inhibits curcumin-induced apoptosis via the prevention of intracellular Ca2+ depletion and cytochrome c release. Biochem Biophys Res Commun. 2003;303:1073–1079. - PubMed
-
- Benomar Y, Naour N, Aubourg A, Bailleux V, Gertler A, Djiane J, et al. Insulin and leptin induce Glut4 plasma membrane translocation and glucose uptake in a human neuronal cell line by a phosphatidylinositol 3-kinase-dependent mechanism. Endocrinology. 2006;147:2550–2556. - PubMed
-
- Berti L, Kellerer M, Capp E, Haring HU. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: evidence for a P13-kinase mediated effect. Diabetologia. 1997;40:606–609. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

