Retrospective analyses of cisplatin-based doublet combination chemotherapy in patients with advanced gastric cancer
- PMID: 20977739
- PMCID: PMC2978206
- DOI: 10.1186/1471-2407-10-583
Retrospective analyses of cisplatin-based doublet combination chemotherapy in patients with advanced gastric cancer
Abstract
Backgrounds: Cisplatin-based chemotherapy, in combination with fluoropyrimidines or taxanes, have demonstrated efficacy against advanced gastric cancer (AGC). This retrospective study was performed with the data obtained from our cancer chemotherapy registry and eight another cancer centers.
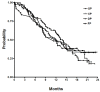
Methods: In 2008, a total of 283 AGC patients were treated with cisplatin-based doublet chemotherapy in the first-line setting: capecitabine plus cisplatin (XP, n = 77), S-1 plus cisplatin (SP, n = 97), taxanes (docetaxel, paclitaxel) plus cisplatin (TP, n = 72), and 5-fluorouracil plus platinum (FP, n = 37). The primary endpoint of this study was overall survival (OS) and the secondary endpoints were safety, response rate and progression-free survival (PFS).
Results: The median age was 54 years with a range of 28-78 years and median delivered number of chemotherapy cycles were XP: 4, SP: 5, TP: 5 and FP: 5, respectively. Objective tumor responses (38%; 95% CI, 32-43%) were 40% for XP, 42% for SP, 36% for DP, and 24% for FP. The estimated median PFS was 4.5 months (95% CI, 3.6-5.4 months) and the median OS was 12.3 months (95% CI, 10.8-13.7 months). No statistically significant difference was found between each regimen used as first-line chemotherapy. At multivariate analysis, independent prognostic parameters for OS were prior gastrectomy, peritoneal dissemination, performance status and hemoglobin level
Conclusion: All of the cisplatin-based doublet chemotherapy regimens appear to be active as first-line chemotherapy for AGC. With better patient selection according to clinical parameters and molecular markers, clinical outcomes of AGC patients in first-line setting can be improved.
Figures
References
-
- Glimelius B, Ekstrom K, Hoffman K, Graf W, Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H. et al.Randomized comparison between chemotherapy plus best supportive care with best supportive care in advanced gastric cancer. Ann Oncol. 1997;8(2):163–168. doi: 10.1023/A:1008243606668. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical