An MLH1 mutation links BACH1/FANCJ to colon cancer, signaling, and insight toward directed therapy
- PMID: 20978114
- PMCID: PMC5358091
- DOI: 10.1158/1940-6207.CAPR-10-0118
An MLH1 mutation links BACH1/FANCJ to colon cancer, signaling, and insight toward directed therapy
Abstract
Defects in MLH1, as with other mismatch repair (MMR) proteins, are the primary cause of hereditary nonpolyposis colon cancer (HNPCC). Mutations in MMR genes often disrupt mismatch repair and MMR signaling functions. However, some HNPCC-associated mutations have unknown pathogenicity. Here, we uncover an MLH1 clinical mutation with a leucine (L)-to-histidine (H) amino acid change at position 607 that ablates MLH1 binding to FANCJ. Given that a DNA helicase is not essential for mammalian MMR in vitro, we considered that loss of MLH1 binding to FANCJ could alter MMR signaling. Consistent with this hypothesis, FANCJ-deficient cells exhibit delayed MMR signaling and apoptotic responses that generate resistance to agents that induce O(6)-methylguanine lesions. Our data indicate that the delay in MMR signaling provides time for the methylguanine methyltransferase (MGMT) enzyme to reverse DNA methylation. In essence, FANCJ deficiency alters the competition between two pathways: MGMT-prosurvival versus MMR-prodeath. This outcome could explain the HNPCC familial cancers that present as microsatellite stable and with intact MMR, such as MLH(L607H). Importantly, the link between FANCJ and HNPCC provides insight toward directed therapies because loss of the FANCJ/MLH1 interaction also uniquely sensitizes cells to DNA cross-linking agents.
©2010 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures
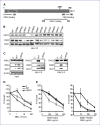

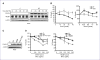


Comment in
-
Making sense of missense in Lynch syndrome: the clinical perspective.Cancer Prev Res (Phila). 2010 Nov;3(11):1371-4. doi: 10.1158/1940-6207.CAPR-10-0204. Epub 2010 Oct 26. Cancer Prev Res (Phila). 2010. PMID: 20978117 Free PMC article.
References
-
- O’Brien V, Brown R. Signalling cell cycle arrest and cell death through the MMR System. Carcinogenesis. 2006;27:682–92. - PubMed
-
- Branch P, Aquilina G, Bignami M, Karran P. Defective mismatch binding and a mutator phenotype in cells tolerant to DNA damage. Nature. 1993;362:652–4. - PubMed
-
- Schofield MJ, Hsieh P. DNA mismatch repair: molecular mechanisms and biological function. Annu Rev Microbiol. 2003;57:579–608. - PubMed
-
- Karran P, Marinus MG. Mismatch correction at O6-methylguanine residues in E. coli DNA. Nature. 1982;296:868–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

