Cancer-associated p53 tetramerization domain mutants: quantitative analysis reveals a low threshold for tumor suppressor inactivation
- PMID: 20978130
- PMCID: PMC3012982
- DOI: 10.1074/jbc.M110.174698
Cancer-associated p53 tetramerization domain mutants: quantitative analysis reveals a low threshold for tumor suppressor inactivation
Abstract
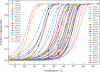
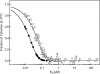
The tumor suppressor p53, a 393-amino acid transcription factor, induces cell cycle arrest and apoptosis in response to genotoxic stress. Its inactivation via the mutation of its gene is a key step in tumor progression, and tetramer formation is critical for p53 post-translational modification and its ability to activate or repress the transcription of target genes vital in inhibiting tumor growth. About 50% of human tumors have TP53 gene mutations; most are missense ones that presumably lower the tumor suppressor activity of p53. In this study, we explored the effects of known tumor-derived missense mutations on the stability and oligomeric structure of p53; our comprehensive, quantitative analyses encompassed the tetramerization domain peptides representing 49 such substitutions in humans. Their effects on tetrameric structure were broad, and the stability of the mutant peptides varied widely (ΔT(m) = 4.8 ∼ -46.8 °C). Because formation of a tetrameric structure is critical for protein-protein interactions, DNA binding, and the post-translational modification of p53, a small destabilization of the tetrameric structure could result in dysfunction of tumor suppressor activity. We suggest that the threshold for loss of tumor suppressor activity in terms of the disruption of the tetrameric structure of p53 could be extremely low. However, other properties of the tetramerization domain, such as electrostatic surface potential and its ability to bind partner proteins, also may be important.
Figures
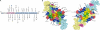
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

