The cytoskeleton and the peroxisomal-targeted snowy cotyledon3 protein are required for chloroplast development in Arabidopsis
- PMID: 20978221
- PMCID: PMC2990128
- DOI: 10.1105/tpc.110.074781
The cytoskeleton and the peroxisomal-targeted snowy cotyledon3 protein are required for chloroplast development in Arabidopsis
Abstract
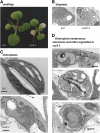
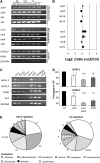
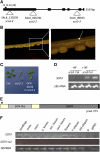
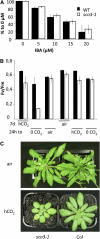
Here, we describe the snowy cotyledon3 (sco3-1) mutation, which impairs chloroplast and etioplast development in Arabidopsis thaliana seedlings. SCO3 is a member of a largely uncharacterized protein family unique to the plant kingdom. The sco3-1 mutation alters chloroplast morphology and development, reduces chlorophyll accumulation, impairs thylakoid formation and photosynthesis in seedlings, and results in photoinhibition under extreme CO(2) concentrations in mature leaves. There are no readily apparent changes to chloroplast biology, such as transcription or assembly that explain the disruption to chloroplast biogenesis. Indeed, SCO3 is actually targeted to another organelle, specifically to the periphery of peroxisomes. However, impaired chloroplast development cannot be attributed to perturbed peroxisomal metabolic processes involving germination, fatty acid β-oxidation or photorespiration, though there are so far undescribed changes in low and high CO(2) sensitivity in seedlings and young true leaves. Many of the chloroplasts are bilobed, and some have persistent membranous extensions that encircle other cellular components. Significantly, there are changes to the cytoskeleton in sco3-1, and microtubule inhibitors have similar effects on chloroplast biogenesis as sco3-1 does. The localization of SCO3 to the periphery of the peroxisomes was shown to be dependent on a functional microtubule cytoskeleton. Therefore, the microtubule and peroxisome-associated SCO3 protein is required for chloroplast development, and sco3-1, along with microtubule inhibitors, demonstrates an unexpected role for the cytoskeleton and peroxisomes in chloroplast biogenesis.
Figures



References
-
- Albrecht V., Ingenfeld A., Apel K. (2006). Characterization of the snowy cotyledon 1 mutant of Arabidopsis thaliana: The impact of chloroplast elongation factor G on chloroplast development and plant vitality. Plant Mol. Biol. 60: 507–518 - PubMed
-
- Albrecht V., Ingenfeld A., Apel K. (2008). Snowy cotyledon 2: The identification of a zinc finger domain protein essential for chloroplast development in cotyledons but not in true leaves. Plant Mol. Biol. 66: 599–608 - PubMed
-
- Asano T., Yoshioka Y., Machida Y. (2004). A defect in atToc159 of Arabidopsis thaliana causes severe defects in leaf development. Genes Genet. Syst. 79: 207–212 - PubMed
-
- Bauer J., Chen K., Hiltbunner A., Wehrli E., Eugster M., Schnell D., Kessler F. (2000). The major protein import receptor of plastids is essential for chloroplast biogenesis. Nature 403: 203–207 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

