Homologous regions of autoantibody heavy chain complementarity-determining region 3 (H-CDR3) in patients with pemphigus cause pathogenicity
- PMID: 20978359
- PMCID: PMC2964997
- DOI: 10.1172/JCI44425
Homologous regions of autoantibody heavy chain complementarity-determining region 3 (H-CDR3) in patients with pemphigus cause pathogenicity
Abstract
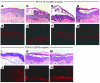
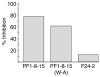
Pemphigus is a life-threatening autoimmune disease in which antibodies specific for desmogleins (Dsgs) cause loss of keratinocyte cell adhesion and blisters. In order to understand how antibodies cause pathogenicity and whether there are commonalities among antibodies in different patients that could ultimately be used to target specific therapy against these antibodies, we characterized Dsg-specific mAbs cloned by phage display from 3 patients with pemphigus vulgaris and 2 with pemphigus foliaceus. Variable heavy chain gene usage was restricted, but similar genes were used for both pathogenic and nonpathogenic mAbs. However, the heavy chain complementarity-determining region 3 (H-CDR3) of most pathogenic, but not nonpathogenic, mAbs shared an amino acid consensus sequence. Randomization of the H-CDR3 and site-directed mutagenesis indicated that changes in this sequence could block pathogenicity but not necessarily binding. In addition, for 2 antibodies with longer H-CDR3s, a tryptophan was critical for pathogenicity but not binding, a result that is consistent with blocking the tryptophan acceptor site that is thought to be necessary for Dsg-mediated adhesion. These studies indicate that H-CDR3 is critical for pathogenicity of a human autoantibody, that a small region (even 1 amino acid) can mediate pathogenicity, and that pathogenicity can be uncoupled from binding in these antibodies.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

