Role of biotransformation in drug-induced toxicity: influence of intra- and inter-species differences in drug metabolism
- PMID: 20978360
- PMCID: PMC4675351
- DOI: 10.2133/dmpk.dmpk-10-rv-089
Role of biotransformation in drug-induced toxicity: influence of intra- and inter-species differences in drug metabolism
Abstract
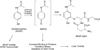
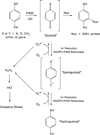
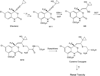
It is now widely appreciated that drug metabolites, in addition to the parent drugs themselves, can mediate the serious adverse effects exhibited by some new therapeutic agents, and as a result, there has been heightened interest in the field of drug metabolism from researchers in academia, the pharmaceutical industry, and regulatory agencies. Much progress has been made in recent years in understanding mechanisms of toxicities caused by drug metabolites, and in understanding the numerous factors that influence individual exposure to products of drug biotransformation. This review addresses some of these factors, including the role of drug-drug interactions, reactive metabolite formation, individual susceptibility, and species differences in drug disposition caused by genetic polymorphisms in drug-metabolizing enzymes. Examples are provided of adverse reactions that are linked to drug metabolism, and the mechanisms underlying variability in toxic response are discussed. Finally, some future directions for research in this field are highlighted in the context of the discovery and development of new therapeutic agents.
Figures


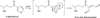
References
-
- Baillie TA. Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism. Chem. Res. Toxicol. 2008;21:129–137. - PubMed
-
- Korfmacher WA. Advances in the integration of drug metabolism into the lead optimization paradigm. Mini-Rev. Med. Chem. 2009;9:703–716. - PubMed
-
- Olson H, Betton G, Robinson D, Thomas K, Monro A, Kolaja G, Lilly P, Sanders J, Sipes G, Bracken W, Dorato M, Van Deun K, Smith P, Berger B, Heller A. Concordance of the toxicity of pharmaceuticals in humans and in animals. Reg. Toxicol. Pharmacol. 2000;32:56–67. - PubMed
-
- Greaves P, Williams A, Eve M. First dose of potential new medicines to humans: how animals help. Nature Rev. Drug Discov. 2004;3:226–236. - PubMed
-
- Peters TS. Do preclinical testing strategies help predict human hepatotoxic potentials? Toxicol. Pathol. 2005;33:146–154. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
