The status of exon skipping as a therapeutic approach to duchenne muscular dystrophy
- PMID: 20978473
- PMCID: PMC3017449
- DOI: 10.1038/mt.2010.219
The status of exon skipping as a therapeutic approach to duchenne muscular dystrophy
Abstract
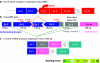
Duchenne muscular dystrophy (DMD) is associated with mutations in the dystrophin gene that disrupt the open reading frame whereas the milder Becker's form is associated with mutations which leave an in-frame mRNA transcript that can be translated into a protein that includes the N- and C- terminal functional domains. It has been shown that by excluding specific exons at, or adjacent to, frame-shifting mutations, open reading frame can be restored to an out-of-frame mRNA, leading to the production of a partially functional Becker-like dystrophin protein. Such targeted exclusion can be achieved by administration of oligonucleotides that are complementary to sequences that are crucial to normal splicing of the exon into the transcript. This principle has been validated in mouse and canine models of DMD with a number of variants of oligonucleotide analogue chemistries and by transduction with adeno-associated virus (AAV)-small nuclear RNA (snRNA) reagents encoding the antisense sequence. Two different oligonucleotide agents are now being investigated in human trials for splicing out of exon 51 with some early indications of success at the biochemical level.
Figures
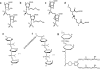

References
-
- Dunckley MG, Manoharan M, Villiet P, Eperon IC., and, Dickson G. Modification of splicing in the dystrophin gene in cultured Mdx muscle cells by antisense oligoribonucleotides. Hum Mol Genet. 1998;7:1083–1090. - PubMed
-
- Alter J, Lou F, Rabinowitz A, Yin H, Rosenfeld J, Wilton SD, et al. Systemic delivery of morpholino oligonucleotide restores dystrophin expression bodywide and improves dystrophic pathology. Nat Med. 2006;12:175–177. - PubMed
-
- Lu QL, Mann CJ, Lou F, Bou-Gharios G, Morris GE, Xue SA, et al. Functional amounts of dystrophin produced by skipping the mutated exon in the mdx dystrophic mouse. Nat Med. 2003;9:1009–1014. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

