Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases
- PMID: 20978535
- PMCID: PMC2957078
- DOI: 10.1128/mBio.00183-10
Specific control of Pseudomonas aeruginosa surface-associated behaviors by two c-di-GMP diguanylate cyclases
Abstract
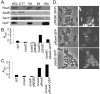
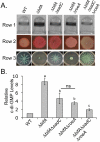
The signaling nucleotide cyclic diguanylate (c-di-GMP) regulates the transition between motile and sessile growth in a wide range of bacteria. Understanding how microbes control c-di-GMP metabolism to activate specific pathways is complicated by the apparent multifold redundancy of enzymes that synthesize and degrade this dinucleotide, and several models have been proposed to explain how bacteria coordinate the actions of these many enzymes. Here we report the identification of a diguanylate cyclase (DGC), RoeA, of Pseudomonas aeruginosa that promotes the production of extracellular polysaccharide (EPS) and contributes to biofilm formation, that is, the transition from planktonic to surface-dwelling cells. Our studies reveal that RoeA and the previously described DGC SadC make distinct contributions to biofilm formation, controlling polysaccharide production and flagellar motility, respectively. Measurement of total cellular levels of c-di-GMP in ∆roeA and ∆sadC mutants in two different genetic backgrounds revealed no correlation between levels of c-di-GMP and the observed phenotypic output with regard to swarming motility and EPS production. Our data strongly argue against a model wherein changes in total levels of c-di-GMP can account for the specific surface-related phenotypes of P. aeruginosa.
Figures



References
-
- Römling U., Gomelsky M., Galperin M. Y. 2005. c-di-GMP: the dawning of a novel bacterial signaling system. Mol. Microbiol. 57:629–639 - PubMed
-
- Miller J. H. 1992. A short course in bacterial genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
-
- Garcia B., Latasa C., Solano C., Garcia-del Portillo F., Gamazo C., Lasa I. 2004. Role of the GGDEF protein family in Salmonella cellulose biosynthesis and biofilm formation. Mol. Microbiol. 54:264–277 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

