Effects of enzyme replacement therapy on growth in patients with mucopolysaccharidosis type II
- PMID: 20978944
- PMCID: PMC3026660
- DOI: 10.1007/s10545-010-9215-2
Effects of enzyme replacement therapy on growth in patients with mucopolysaccharidosis type II
Abstract
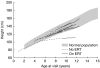
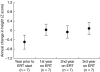
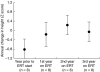
Mucopolysaccharidosis type II (MPS II; Hunter syndrome) is an X-linked, recessive, lysosomal storage disorder caused by deficiency of iduronate-2-sulfatase. It has multisystemic involvement, with manifestations in the brain, upper respiratory tract, heart, abdomen, joints and bones. Bone involvement leads to decreased growth velocity and short stature in nearly all patients. A therapeutic option for patients with MPS II is enzyme replacement therapy (ERT) with idursulfase (Elaprase®). We compared annual growth rates before and during ERT in 18 patients from Mainz, Germany, and Manchester, UK. Group 1 included nine patients who started ERT before 10 years of age; group 2 contained nine patients aged more than 10 years at the start of ERT. All patients had received weekly or biweekly ERT or placebo for 1 year, followed by ERT for more than 3 years. For patients in group 1, the mean (± SD) height increase was 14.6 ± 5.5 cm during 3 years of ERT. Only one patient in this group (who was below the 3rd percentile when starting ERT) deviated from the normal growth curve over this time. Patients in group 2 had a mean height increase of 8.1 ± 1.7 cm after 3 years of ERT compared with an increase of 1 cm in the year before ERT. ERT seems to have a positive influence on growth in patients with MPS II. Most benefit is seen in patients beginning ERT before the age of 10 years. This supports the recommendation that ERT should be started as early as possible in patients with MPS II.
Figures





References
-
- Beck M, Muenzer J, Scarpa M, Schulze Frenking G, Wraith E, on behalf of the HOS investigators (2008) Effect of enzyme replacement therapy on growth in patients with Hunter syndrome: results from the Hunter Outcome Survey. Abstract presented at the American Society of Human Genetics 2008. http://www.ashg.org/2008meeting/abstracts/fulltext/f20738.htm. Accessed January 2010

