Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells
- PMID: 20979056
- PMCID: PMC3505073
- DOI: 10.1002/hep.23999
Human T cell microparticles circulate in blood of hepatitis patients and induce fibrolytic activation of hepatic stellate cells
Abstract
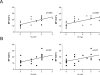
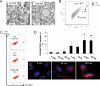
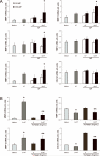
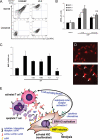
Microparticles (MPs) are small cell membrane vesicles that are released from cells during apoptosis or activation. Although circulating platelet MPs have been studied in some detail, the existence and functional role of T cell MPs remain elusive. We show that blood from patients with active hepatitis C (alanine aminotransferase [ALT] level >100 IU/mL) contains elevated numbers of T cell MPs compared with patients with mild hepatitis C (ALT <40 IU/mL) and healthy controls. T cell MPs fuse with cell membranes of hepatic stellate cells (HSCs), the major effector cells for excess matrix deposition in liver fibrosis and cirrhosis. MP uptake is partly intercellular adhesion molecule 1-dependent and leads to activation of nuclear factor kappa B and extracellular signal-regulated kinases 1 and 2 and subsequent up-regulation of fibrolytic genes in HSCs, down-regulation of procollagen α1(I) messenger RNA, and blunting of profibrogenic activities of transforming growth factor β1. Ex vivo, the induced fibrolytic activity is evident in MPs derived from activated CD4+ T cells and is highest in MPs derived from activated and apoptotic CD8+ T cells. Mass spectrometry, fluorescence-activated cell sorting analysis, and function blocking antibodies revealed CD147/Emmprin as a candidate transmembrane molecule in HSC fibrolytic activation by CD8+ T cell MPs.
Conclusion: Circulating T cell MPs are a novel diagnostic marker for inflammatory liver diseases, and in vivo induction of T cell MPs may be a novel strategy to induce regression of liver fibrosis.
Copyright © 2010 American Association for the Study of Liver Diseases.
Figures


References
-
- Dienstag JL, Goldin RD, Heathcote EJ, Hann HW, Woessner M, Stephenson SL, Gardner S, et al. Histological outcome during long-term lamivudine therapy. Gastroenterology. 2003;124:105–117. - PubMed
-
- Issa R, Zhou X, Constandinou CM, Fallowfield J, Millward-Sadler H, Gaca MD, Sands E, et al. Spontaneous recovery from micronodular cirrhosis: evidence for incomplete resolution associated with matrix cross-linking. Gastroenterology. 2004;126:1795–1808. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
