Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults
- PMID: 20979459
- PMCID: PMC3107551
- DOI: 10.1086/657158
Circulating antibody-secreting cells during acute respiratory syncytial virus infection in adults
Abstract
Background: The specificity and duration of circulating human antibody-secreting cells (ASCs) after vaccination have been well described, but characteristics of ASCs during acute respiratory infections have not been well studied.
Methods: Circulating antigen-specific ASCs were measured at 3 time points (enrollment, days 10-16, and days 22-45) in 40 adults during respiratory syncytial virus (RSV) infection.
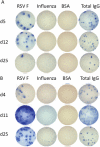
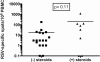
Results: Of the 40 patients, 36 (90%) had detectable circulating RSV F protein-specific ASCs within 11 days after illness onset. The magnitude of the RSV-specific ASCs was 1-1500 spots per 10⁶ peripheral blood mononuclear cells (mean frequency [± standard deviation], 200 ± 256 spots per 10⁶ peripheral blood mononuclear cells). ASCs were detected on day 8-16 and day 22-45 after symptom onset in 78% and 48% of subjects, respectively. Subjects shedding virus for >10 days were more likely to have a positive response to ASC enzyme-linked immunospot assay at the late time point than those shedding for ≤10 days (8 of 12 subjects vs 2 of 11 subjects; P = .02).
Conclusions: The kinetics of ASC circulation during acute mucosal viral infections was more prolonged than that we had observed after a single intramuscular injection with inactivated influenza vaccine in a study reported elsewhere. The association between the duration of virus shedding and the persistence of detectable viral-specific ASCs suggests that ongoing antigen persistence induces a prolonged temporal pattern of ASC generation.
Conflict of interest statement
Potential conflicts of interest: F.E.-H.L. has research grants from Trellis Bioscience. A.R.F. serves on the advisory board of Quidel and has done consulting work for AstraZeneca, MedImmune, and Wyeth. I.S. has done consulting work for Genentech and Biogen. E.E.W. has consulted for AstraZeneca and Boehringer Ingelheim. A.R.F. and E.E.W. have research grants from GlaxoSmithKline and Sanofi Pasteur. J.L.H. reports no conflicts of interest.
Figures




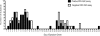
References
-
- Hall CB. Respiratory syncytial virus and parainfluenza virus. N Engl JMed. 2001;344:1917–1928. - PubMed
-
- Falsey AR, Hennessey PA, Formica MA, ox C, Walsh EE. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med. 2005;352:1749–1759. - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140:543–546. - PubMed
-
- Hall CB, Walsh EE, Long CE, Schnabel KC. Immunity to and frequency of reinfection with respiratory syncytial virus. J Infect Dis. 1991;163:693–698. - PubMed
-
- Graham BS. Immunological determinants of disease caused by respiratory syncytial virus. Trends Microbiol. 1996;4:290–293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

