Heme oxygenase-1 prevents non-alcoholic steatohepatitis through suppressing hepatocyte apoptosis in mice
- PMID: 20979658
- PMCID: PMC2984485
- DOI: 10.1186/1476-511X-9-124
Heme oxygenase-1 prevents non-alcoholic steatohepatitis through suppressing hepatocyte apoptosis in mice
Erratum in
-
Correction: Heme oxygenase-1 prevents non-alcoholic steatohepatitis through suppressing hepatocyte apoptosis in mice.Lipids Health Dis. 2025 Dec 23;24(1):387. doi: 10.1186/s12944-025-02816-y. Lipids Health Dis. 2025. PMID: 41437355 Free PMC article. No abstract available.
Abstract
Objective: Heme oxygenase-1 (HO-1), the rate-limiting enzyme in heme catabolism, has been reported to have potential antioxidant properties. However, the role of HO-1 on hepatocyte apoptosis remains unclear. We aim to elucidate the effects of HO-1 on oxidative stress related hepatocellular apoptosis in nutritional steatohepatitis in mice.
Methods: C57BL/6J mice were fed with methionine-choline deficient (MCD) diet for four weeks to induce hepatic steatohepatitis. HO-1 chemical inducer (hemin), HO-1 chemical inhibitor zinc protoporphyrin IX (ZnPP-IX) and/or adenovirus carrying HO-1 gene (Ad-HO-1) were administered to mice, respectively. Hepatocyte apoptosis was evaluated by terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay, the mRNA and protein expression of apoptosis related genes were assayed by quantitative real-time PCR and Western blot.
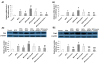
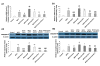
Results: Hepatocyte signs of oxidative related apoptotic injury were presented in mice fed with MCD diet for 4 weeks. Induction of HO-1 by hemin or Ad-HO-1 significantly attenuated the severity of liver histology, which was associated with decreased hepatic lipid peroxidation content, reduced number of apoptotic cells by TUNEL staining, down-regulated expression of pro-apoptosis related genes including Fas/FasL, Bax, caspase-3 and caspase-9, reduced expression of cytochrome p4502E1 (CYP2E1), inhibited cytochrome c (Cyt-c) release, and up-regulated expression of anti-apoptosis gene Bcl-2. Whereas, inhibition of HO-1 by ZnPP-IX caused oxidative stress related hepatic injury, which concomitant with increased number of TUNEL positive cells and up-regulated expression of pro-apoptosis related genes.
Conclusions: The present study provided evidences for the protective role of HO-1 in preventing nutritional steatohepatitis through suppressing hepatocyte apoptosis in mice.
Figures

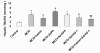



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

