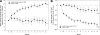Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study
- PMID: 20980427
- PMCID: PMC2963502
- DOI: 10.2337/dc10-0493
Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12-month, randomized, controlled, double-masked, multicenter phase II study
Abstract
Objective: The expression of vascular endothelial growth factor (VEGF) is elevated in diabetic macular edema (DME). Ranibizumab binds to and inhibits multiple VEGF variants. We investigated the safety and efficacy of ranibizumab in DME involving the foveal center.
Research design and methods: This was a 12-month, multicenter, sham-controlled, double-masked study with eyes (age>18 years, type 1 or 2 diabetes, central retinal thickness [CRT]≥300 μm, and best corrected visual acuity [BCVA] of 73-39 ETDRS letters [Early Treatment Diabetic Retinopathy Study]) randomly assigned to intravitreal ranibizumab (0.3 or 0.5 mg; n=51 each) or sham (n=49). The treatment schedule comprised three monthly injections, after which treatment could be stopped/reinitiated with an opportunity for rescue laser photocoagulation (protocol-defined criteria). After month 1, dose-doubling was permitted (protocol-defined criteria, injection volume increased from 0.05 to 0.1 ml and remained at 0.1 ml thereafter). Efficacy (BCVA and CRT) and safety were compared between pooled ranibizumab and sham arms using the full analysis set (n=151, patients receiving≥1 injection).
Results: At month 12, mean±SD BCVA improved from baseline by 10.3±9.1 letters with ranibizumab and declined by 1.4±14.2 letters with sham (P<0.0001). Mean CRT reduction was 194.2±135.1 μm with ranibizumab and 48.4±153.4 μm with sham (P<0.0001). Gain of ≥10 letters BCVA from baseline occurred in 60.8% of ranibizumab and 18.4% of sham eyes (P<0.0001). Safety data were consistent with previous studies of intravitreal ranibizumab.
Conclusions: Ranibizumab is effective in improving BCVA and is well tolerated in DME. Future clinical trials are required to confirm its long-term efficacy and safety.
Trial registration: ClinicalTrials.gov NCT00284050.
Figures
Comment in
-
Targeting the pathophysiology of diabetic macular edema.Diabetes Care. 2010 Nov;33(11):2484-5. doi: 10.2337/dc10-1580. Diabetes Care. 2010. PMID: 20980428 Free PMC article. No abstract available.
References
-
- Diabetes. Fact sheet No. 312 [article online], 2009. Available from http://www.who.int/mediacentre/factsheets/fs312/en/ Accessed 20 December 2009
-
- Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy: XVII. The 14-year incidence and progression of diabetic retinopathy and associated risk factors in type 1 diabetes. Ophthalmology 1998;105:1801–1815 - PubMed
-
- King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care 1998;21:1414–1431 - PubMed
-
- Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol 1985;103:1796–806 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials