The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential
- PMID: 20980457
- PMCID: PMC3028360
- DOI: 10.2337/db10-0403
The protective role of Smad7 in diabetic kidney disease: mechanism and therapeutic potential
Abstract
Objective: Although Smad3 has been considered as a downstream mediator of transforming growth factor-β (TGF-β) signaling in diabetes complications, the role of Smad7 in diabetes remains largely unclear. The current study tests the hypothesis that Smad7 may play a protective role and has therapeutic potential for diabetic kidney disease.
Research design and methods: Protective role of Smad7 in diabetic kidney disease was examined in streptozotocin-induced diabetic mice that have Smad7 gene knockout (KO) and in diabetic rats given Smad7 gene transfer using an ultrasound-microbubble-mediated technique.
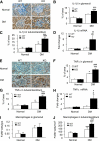
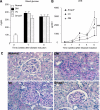
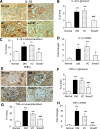
Results: We found that mice deficient for Smad7 developed more severe diabetic kidney injury than wild-type mice as evidenced by a significant increase in microalbuminuria, renal fibrosis (collagen I, IV, and fibronectin), and renal inflammation (interleukin-1β [IL-1β], tumor necrosis factor-α [TNF-α], monocyte chemoattractant protein-1 [MCP-1], intracellular adhesion molecule-1 [ICAM-1], and macrophages). Further studies revealed that enhanced renal fibrosis and inflammation in Smad7 KO mice with diabetes were associated with increased activation of both TGF-β/Smad2/3 and nuclear factor-κB (NF-κB) signaling pathways. To develop a therapeutic potential for diabetic kidney disease, Smad7 gene was transferred into the kidney in diabetic rats by an ultrasound-microbubble-mediated technique. Although overexpression of renal Smad7 had no effect on levels of blood glucose, it significantly attenuated the development of microalbuminuria, TGF-β/Smad3-mediated renal fibrosis such as collagen I and IV and fibronectin accumulation and NF-κB/p65-driven renal inflammation including IL-1β, TNF-α, MCP-1, and ICAM-1 expression and macrophage infiltration in diabetic rats.
Conclusions: Smad7 plays a protective role in diabetic renal injury. Overexpression of Smad7 may represent a novel therapy for the diabetic kidney complication.
Figures





References
-
- Caramori ML, Kim Y, Huang C, et al. Cellular basis of diabetic nephropathy: 1. Study design and renal structural-functional relationships in patients with long-standing type 1 diabetes. Diabetes 2002;51:506–513 - PubMed
-
- White KE, Bilous RW. Type 2 diabetic patients with nephropathy show structural-functional relationships that are similar to type 1 disease. J Am Soc Nephrol 2000;11:1667–1673 - PubMed
-
- Finne P, Reunanen A, Stenman S, Groop PH, Grönhagen-Riska C. Incidence of end-stage renal disease in patients with type 1 diabetes. JAMA 2005;294:1782–1787 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

