A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas
- PMID: 20980506
- PMCID: PMC3014199
- DOI: 10.1128/JVI.01512-10
A new model of Epstein-Barr virus infection reveals an important role for early lytic viral protein expression in the development of lymphomas
Abstract
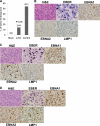
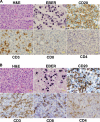
Epstein-Barr virus (EBV) infects cells in latent or lytic forms, but the role of lytic infection in EBV-induced lymphomas is unclear. Here, we have used a new humanized mouse model, in which both human fetal CD34(+) hematopoietic stem cells and thymus/liver tissue are transplanted, to compare EBV pathogenesis and lymphoma formation following infection with a lytic replication-defective BZLF1-deleted (Z-KO) virus or a lytically active BZLF1(+) control. Both the control and Z-KO viruses established long-term viral latency in all infected animals. The infection appeared well controlled in some animals, but others eventually developed CD20(+) diffuse large B cell lymphomas (DLBCL). Animals infected with the control virus developed tumors more frequently than Z-KO virus-infected animals. Specific immune responses against EBV-infected B cells were generated in mice infected with either the control virus or the Z-KO virus. In both cases, forms of viral latency (type I and type IIB) were observed that are less immunogenic than the highly transforming form (type III) commonly found in tumors of immunocompromised hosts, suggesting that immune pressure contributed to the outcome of the infection. These results point to an important role for lytic EBV infection in the development of B cell lymphomas in the context of an active host immune response.
Figures








References
-
- Bandobashi, K., A. Liu, N. Nagy, L. L. Kis, J. Nishikawa, M. Bjorkholm, G. Klein, and E. Klein. 2005. EBV infection induces expression of the transcription factors ATF-2/c-Jun in B lymphocytes but not in B-CLL cells. Virus Genes 30:323-330. - PubMed
-
- Chapman, A. L., and A. B. Rickinson. 1998. Epstein-Barr virus in Hodgkin's disease. Ann. Oncol. 9(Suppl. 5):S5-S16. - PubMed
-
- Cocco, M., C. Bellan, R. Tussiwand, D. Corti, E. Traggiai, S. Lazzi, S. Mannucci, L. Bronz, N. Palummo, C. Ginanneschi, P. Tosi, A. Lanzavecchia, M. G. Manz, and L. Leoncini. 2008. CD34+ cord blood cell-transplanted Rag2−/− γc−/− mice as a model for Epstein-Barr virus infection. Am. J. Pathol. 173:1369-1378. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

