Naked dense bodies provoke depression
- PMID: 20980589
- PMCID: PMC6634796
- DOI: 10.1523/JNEUROSCI.2495-10.2010
Naked dense bodies provoke depression
Abstract
At presynaptic active zones (AZs), the frequently observed tethering of synaptic vesicles to an electron-dense cytomatrix represents a process of largely unknown functional significance. Here, we identified a hypomorphic allele, brpnude, lacking merely the last 1% of the C-terminal amino acids (17 of 1740) of the active zone protein Bruchpilot. In brpnude, electron-dense bodies were properly shaped, though entirely bare of synaptic vesicles. While basal glutamate release was unchanged, paired-pulse and sustained stimulation provoked depression. Furthermore, rapid recovery following sustained release was slowed. Our results causally link, with intramolecular precision, the tethering of vesicles at the AZ cytomatrix to synaptic depression.
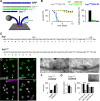
Figures


References
-
- Atwood HL, Karunanithi S. Diversification of synaptic strength: presynaptic elements. Nat Rev Neurosci. 2002;3:497–516. - PubMed
-
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. - PubMed
-
- Dietzl G, Chen D, Schnorrer F, Su KC, Barinova Y, Fellner M, Gasser B, Kinsey K, Oppel S, Scheiblauer S, Couto A, Marra V, Keleman K, Dickson BJ. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature. 2007;448:151–156. - PubMed
-
- Guo W, Sacher M, Barrowman J, Ferro-Novick S, Novick P. Protein complexes in transport vesicle targeting. Trends Cell Biol. 2000;10:251–255. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
