The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats
- PMID: 20980596
- PMCID: PMC3096060
- DOI: 10.1523/JNEUROSCI.2167-10.2010
The parafascicular thalamic nucleus concomitantly influences behavioral flexibility and dorsomedial striatal acetylcholine output in rats
Abstract
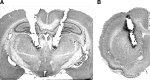
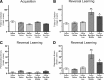
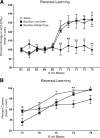
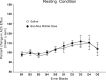
Recent evidence suggests that a circuit involving the centromedian-parafascicular (Pf) thalamus and basal ganglia is critical for a shift away from biased actions. In particular, excitatory input from the Pf onto striatal cholinergic neurons may facilitate behavioral flexibility. Accumulating evidence indicates that an endogenous increase in dorsomedial striatal acetylcholine (ACh) output enhances behavioral flexibility. The present experiments investigated whether the rat (Rattus norvegicus) Pf supports flexibility during reversal learning, in part, by modifying dorsomedial striatal ACh output. This was determined first by examining the effects of Pf inactivation, through infusion of the GABA agonists baclofen and muscimol, on place acquisition and reversal learning. Additional experiments examined Pf inactivation on dorsomedial striatal ACh output during reversal learning and a resting condition. Behavioral testing was performed in a cross-maze. In vivo microdialysis combined with HPLC/electrochemical detection was used to sample ACh from the dorsomedial striatum. Pf inactivation selectively impaired reversal learning in a dose-dependent manner. A subsequent study showed that an increase in dorsomedial striatal ACh efflux (∼30% above basal levels) during reversal learning was blocked by Pf inactivation, which concomitantly impaired reversal learning. In the resting condition, a dose of baclofen and muscimol that blocked a behaviorally induced increase in dorsomedial striatal ACh output did not reduce basal ACh efflux. Together, the present findings indicate that the Pf is an intralaminar thalamic nucleus critical for behavioral flexibility, in part, by directly affecting striatal ACh output under conditions that require a shift in choice patterns.
Figures
References
-
- Apicella P, Scarnati E, Schultz W. Tonically discharging neurons of monkey striatum respond to preparatory and rewarding stimuli. Exp Brain Res. 1991;84:672–675. - PubMed
-
- Apicella P, Scarnati E, Ljungberg T, Schultz W. Neuronal activity in monkey striatum related to the expectation of predictable environmental events. J Neurophysiol. 1992;68:945–960. - PubMed
-
- Bailey KR, Mair RG. Lesions of specific and nonspecific thalamic nuclei affect prefrontal cortex-dependent aspects of spatial working memory. Behav Neurosci. 2005;119:410–419. - PubMed
-
- Baldi G, Russi G, Nannini L, Vezzani A, Consolo S. Trans-synaptic modulation of striatal ACh release in vivo by the parafascicular thalamic nucleus. Eur J Neurosci. 1995;7:1117–1120. - PubMed
-
- Balleine BW, Liljeholm M, Ostlund SB. The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res. 2009;199:43–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
