Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly
- PMID: 20980605
- PMCID: PMC2976578
- DOI: 10.1523/JNEUROSCI.1698-10.2010
Oligodendrocyte myelin glycoprotein does not influence node of ranvier structure or assembly
Abstract
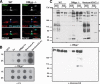
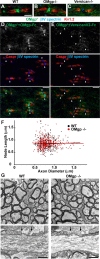
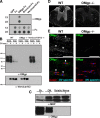
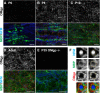
Oligodendrocyte myelin glycoprotein (OMgp) is expressed by both neurons and oligodendrocytes in the CNS. It has been implicated in growth cone collapse and neurite outgrowth inhibition by signaling through the Nogo receptor and paired Ig-like receptor B (PirB). OMgp was also reported to be an extracellular matrix (ECM) protein surrounding CNS nodes of Ranvier and proposed to function as (1) an inhibitor of nodal collateral sprouting and (2) an important contributor to proper nodal and paranodal architecture. However, we show here that the anti-OMgp antiserum used in previous studies to define the functions of OMgp at nodes is not specific. Among all reported nodal ECM components, the antiserum exhibited strong cross-reactivity against versican V2 isoform, a chondroitin sulfate proteoglycan. Furthermore, the OMgp antiserum labeled OMgp-null nodes, but not nodes from versican V2-deficient mice, and preadsorption of the OMgp antiserum with recombinant versican V2 blocked nodal labeling. Analysis of CNS nodes in OMgp-null mice failed to reveal any nodal or paranodal defects, or increased nodal collateral sprouting, indicating that OMgp does not participate in CNS node of Ranvier assembly or maintenance. We successfully identified a highly specific anti-OMgp antibody and observed OMgp staining in white matter only after initiation of myelination. OMgp immunoreactivity decorated the surface of mature myelinated axons, but was excluded from compact myelin and nodes. Together, our results strongly argue against the nodal localization of OMgp and its proposed functions at nodes, and reveal OMgp's authentic localization relative to nodes and myelin.
Figures
References
-
- Apostolski S, Sadiq SA, Hays A, Corbo M, Suturkova-Milosevic L, Chaliff P, Stefansson K, LeBaron RG, Ruoslahti E, Hays AP, Latov N. Identification of Gal(β1–3)GalNAc bearing glycoproteins at the nodes of Ranvier in peripheral nerve. J Neurosci Res. 1994;38:134–141. - PubMed
-
- Atwal JK, Pinkston-Gosse J, Syken J, Stawicki S, Wu Y, Shatz C, Tessier-Lavigne M. PirB is a functional receptor for myelin inhibitors of axonal regeneration. Science. 2008;322:967–970. - PubMed
-
- Bartsch U, Pesheva P, Raff M, Schachner M. Expression of janusin (J1–160/180) in the retina and optic nerve of the developing and adult mouse. Glia. 1993;9:57–69. - PubMed
-
- Berghs S, Aggujaro D, Dirkx R, Jr, Maksimova E, Stabach P, Hermel JM, Zhang JP, Philbrick W, Slepnev V, Ort T, Solimena M. betaIV spectrin, a new spectrin localized at axon initial segments and nodes of Ranvier in the central and peripheral nervous system. J Cell Biol. 2000;151:985–1002. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
