Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment
- PMID: 20980612
- PMCID: PMC2989851
- DOI: 10.1523/JNEUROSCI.3037-10.2010
Locus ceruleus and anterior cingulate cortex sustain wakefulness in a novel environment
Abstract
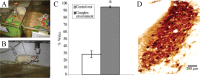
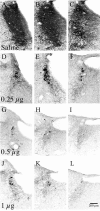
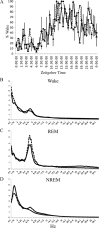
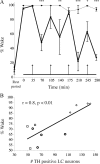
Locus ceruleus (LC) neuronal activity is correlated with the waking state, yet LC lesions produce only minor alterations in daily wakefulness. Here, we report that sustained elevations in neurobehavioral and EEG arousal in response to exposure to an environment with novel stimuli, including social interaction, are prevented by selective chemical lesions of the LC in rats. Similar results are seen when the anterior cingulate cortex (ACC), which receives especially dense LC innervation, is selectively denervated of LC input or is ablated by the cell-specific neurotoxin ibotenic acid. Anterograde tracing combined with tyrosine hydroxylase immunohistochemistry demonstrates ACC terminals in apposition with the distal dendrites of LC neurons. Our data implicate the ACC as both a source of input to the LC as well as one of its targets and suggests that the two structures engage in a dialog that may provide a critical neurobiological substrate for sustained attention.
Figures

References
-
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. - PubMed
-
- Aston-Jones G, Ennis M, Pieribone VA, Nickell WT, Shipley MT. The brain nucleus locus coeruleus: restricted afferent control of a broad efferent network. Science. 1986;234:734–737. - PubMed
-
- Berridge CW, España RA. Synergistic sedative effects of noradrenergic alpha(1)- and beta-receptor blockade on forebrain electroencephalographic and behavioral indices. Neuroscience. 2000;99:495–505. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
