Characterization of purified human Bact spliceosomal complexes reveals compositional and morphological changes during spliceosome activation and first step catalysis
- PMID: 20980672
- PMCID: PMC2995400
- DOI: 10.1261/rna.2456210
Characterization of purified human Bact spliceosomal complexes reveals compositional and morphological changes during spliceosome activation and first step catalysis
Abstract
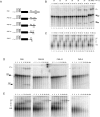
To better understand the compositional and structural dynamics of the human spliceosome during its activation, we set out to isolate spliceosomal complexes formed after precatalytic B but prior to catalytically active C complexes. By shortening the polypyrimidine tract of the PM5 pre-mRNA, which lacks a 3' splice site and 3' exon, we stalled spliceosome assembly at the activation stage. We subsequently affinity purified human B(act) complexes under the same conditions previously used to isolate B and C complexes, and analyzed their protein composition by mass spectrometry. A comparison of the protein composition of these complexes allowed a fine dissection of compositional changes during the B to B(act) and B(act) to C transitions, and comparisons with the Saccharomyces cerevisiae B(act) complex revealed that the compositional dynamics of the spliceosome during activation are largely conserved between lower and higher eukaryotes. Human SF3b155 and CDC5L were shown to be phosphorylated specifically during the B to B(act) and B(act) to C transition, respectively, suggesting these modifications function at these stages of splicing. The two-dimensional structure of the human B(act) complex was determined by electron microscopy, and a comparison with the B complex revealed that the morphology of the human spliceosome changes significantly during its activation. The overall architecture of the human and S. cerevisiae B(act) complex is similar, suggesting that many of the higher order interactions among spliceosomal components, as well as their dynamics, are also largely conserved.
Figures
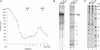




References
-
- Bernstein HS, Coughlin SR 1997. Pombe Cdc5-related protein. A putative human transcription factor implicated in mitogen-activated signaling. J Biol Chem 272: 5833–5837 - PubMed
-
- Bernstein HS, Coughlin SR 1998. A mammalian homolog of fission yeast Cdc5 regulates G2 progression and mitotic entry. J Biol Chem 273: 4666–4671 - PubMed
-
- Bessonov S, Anokhina M, Will CL, Urlaub H, Lührmann R 2008. Isolation of an active step I spliceosome and composition of its RNP core. Nature 452: 846–850 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
