Comparison of treatment and outcome information between a clinical trial and the National Cancer Data Repository
- PMID: 20981742
- PMCID: PMC11439996
- DOI: 10.1002/bjs.7295
Comparison of treatment and outcome information between a clinical trial and the National Cancer Data Repository
Abstract
Background: Clinical trials are important but many factors limit their success, including the costs of long-term follow-up and participants often not being representative of the general population. The National Cancer Data Repository (NCDR) contains data about patients with cancer in England that may help overcome some of these problems. This study compared treatment and outcome information between the Medical Research Council Conventional versus Laparoscopic-Assisted Surgery in Colorectal Cancer (CLASICC) trial and the NCDR.
Methods: Participants in the CLASICC trial were identified in the NCDR, and management and outcome data were compared. Data on all surgically treated English patients with colorectal cancer were extracted from the NCDR and compared with those of CLASICC participants.
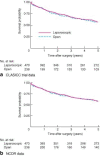
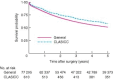
Results: Survival and treatment data for those in the CLASICC trial were available in the NCDR for 98·9 and 95·8 per cent of patients respectively. There was agreement in operation type for 86·1 per cent of patients but surgical approach coding was poor, with only 58·4 per cent of laparoscopic procedures coded in the NCDR. There was no significant difference in survival calculated from either data set. Surgical information was available in the NCDR for 19 of 20 trial participants with missing data. The trial population was younger (P < 0·001), of better socioeconomic status (P = 0·001) and with earlier disease (P < 0·001) than the general surgically treated colorectal cancer population. Rectal cancer survival was similar, but 5-year survival after treatment of colonic cancer was significantly better in the trial than in the national data: 57·1 (95 per cent confidence interval 51·5 to 62·3) versus 49·8 (49·3 to 50·2) per cent respectively.
Conclusion: The National Cancer Data Repository demonstrates potential for informing clinical trials, but limitations prevent full intention-to-treat analyses.
Figures


References
-
- Prescott RJ, Counsell CE, Gillespie WJ, Grant AM, Russell IT, Kiauka S et al. Factors that limit the quality, number and progress of randomised controlled trials. Health Technol Assess 1999; 3: 1–143. - PubMed
-
- Cottin V, Arpin D, Lasset C, Cordier JF, Brune J, Chauvin F et al. Small-cell lung cancer: patients included in clinical trials are not representative of the patient population as a whole. Ann Oncol 1999; 10: 809–815. - PubMed
-
- Kalata P, Martus P, Zettl H, Rodel C, Hohenberger W, Raab R et al. ; German Rectal Cancer Study Group . Differences between clinical trial participants and patients in a population-based registry: the German Rectal Cancer Study vs. the Rostock Cancer Registry. Dis Colon Rectum 2009; 52: 425–437. - PubMed
-
- Peppercorn JM, Weeks JC, Cook EF, Joffe S. Comparison of outcomes in cancer patients treated within and outside clinical trials: conceptual framework and structured review. Lancet 2004; 363: 263–270. - PubMed
-
- Masoudi FA, Havranek EP, Wolfe P, Gross CP, Rathmore SS, Steiner JF et al. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J 2003; 146: 250–257. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

