CD and MCD spectroscopic studies of the two Dps miniferritin proteins from Bacillus anthracis: role of O2 and H2O2 substrates in reactivity of the diiron catalytic centers
- PMID: 21028901
- PMCID: PMC3075618
- DOI: 10.1021/bi101346c
CD and MCD spectroscopic studies of the two Dps miniferritin proteins from Bacillus anthracis: role of O2 and H2O2 substrates in reactivity of the diiron catalytic centers
Abstract
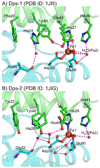
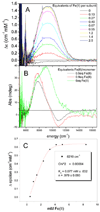
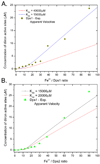
DNA protection during starvation (Dps) proteins are miniferritins found in bacteria and archaea that provide protection from uncontrolled Fe(II)/O radical chemistry; thus the catalytic sites are targets for antibiotics against pathogens, such as anthrax. Ferritin protein cages synthesize ferric oxymineral from Fe(II) and O(2)/H(2)O(2), which accumulates in the large central cavity; for Dps, H(2)O(2) is the more common Fe(II) oxidant contrasting with eukaryotic maxiferritins that often prefer dioxygen. To better understand the differences in the catalytic sites of maxi- versus miniferritins, we used a combination of NIR circular dichroism (CD), magnetic circular dichroism (MCD), and variable-temperature, variable-field MCD (VTVH MCD) to study Fe(II) binding to the catalytic sites of the two Bacillus anthracis miniferritins: one in which two Fe(II) react with O(2) exclusively (Dps1) and a second in which both O(2) or H(2)O(2) can react with two Fe(II) (Dps2). Both result in the formation of iron oxybiomineral. The data show a single 5- or 6-coordinate Fe(II) in the absence of oxidant; Fe(II) binding to Dps2 is 30× more stable than Dps1; and the lower limit of K(D) for binding a second Fe(II), in the absence of oxidant, is 2-3 orders of magnitude weaker than for the binding of the single Fe(II). The data fit an equilibrium model where binding of oxidant facilitates formation of the catalytic site, in sharp contrast to eukaryotic M-ferritins where the binuclear Fe(II) centers are preformed before binding of O(2). The two different binding sequences illustrate the mechanistic range possible for catalytic sites of the family of ferritins.
Figures





References
-
- Lewin AC, Moore GR, Le Brun NE. Formation of protein-coated iron minerals. Dalton Trans. 2005;21:3597–3610. - PubMed
-
- Su M, Cavallo S, Stefanini S, Chiancone E, Chasteen ND. The So-Called Listeria innocua Ferritin Is a Dps Protein. Iron Incorporation, Detoxification, and DNA Protection Properties. Biochemistry. 2005;44:5572–5578. - PubMed
-
- Liu X, Kim K, Leighton T, Theil EC. Paired Bacillus anthracis Dps (mini-ferritin) have different reactivities with peroxide. J. Biol. Chem. 2006;281:27827–27835. - PubMed
-
- Chiancone E, Ceci P. The multifaceted capacity of Dps proteins to combat bacterial stress conditions: Detoxification of iron and hydrogen peroxide and DNA binding. Biochimica et biophysica acta. 2010;1800:798–805. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

