Bis(μ-oxo) dicopper(III) species of the simplest peralkylated diamine: enhanced reactivity toward exogenous substrates
- PMID: 21028910
- PMCID: PMC2993838
- DOI: 10.1021/ic101515g
Bis(μ-oxo) dicopper(III) species of the simplest peralkylated diamine: enhanced reactivity toward exogenous substrates
Abstract
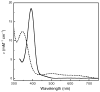
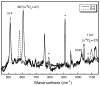
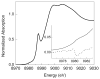
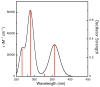
N,N,N',N'-tetramethylethylenediamine (TMED), the simplest and most extensively used peralkylated diamine ligand, is conspicuously absent from those known to form a bis(μ-oxo)dicopper(III) (O) species, [(TMED)(2)Cu(III)(2)(μ(2)-O)(2)](2+), upon oxygenation of its Cu(I) complex. Presented here is the characterization of this O species and its reactivity toward exogenous substrates. Its formation is complicated both by the instability of the [(TMED)Cu(I)](1+) precursor and by competitive formation of a presumed mixed-valent trinuclear [(TMED)(3)Cu(III)Cu(II)(2)(μ(3)-O)(2)](3+) (T) species. Under most reaction conditions, the T species dominates, yet, the O species can be formed preferentially (>80%) upon oxygenation of acetone solutions, if the copper concentration is low (<2 mM) and [(TMED)Cu(I)](1+) is prepared immediately before use. The experimental data of this simplest O species provide a benchmark by which to evaluate density functional theory (DFT) computational methods for geometry optimization and spectroscopic predictions. The enhanced thermal stability of [(TMED)(2)Cu(III)(2)(μ(2)-O)(2)](2+) and its limited steric demands compared to other O species allows more efficient oxidation of exogenous substrates, including benzyl alcohol to benzaldehyde (80% yield), highlighting the importance of ligand structure to not only enhance the oxidant stability but also maintain accessibility to the nascent metal/O(2) oxidant.
Figures
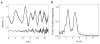
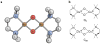
References
-
- Mirica LM, Ottenwaelder X, Stack TDP. Chem Rev. 2004;104:1013–1045. - PubMed
-
- Lewis EA, Tolman WB. Chem Rev. 2004;104:1047–1076. - PubMed
-
- Karlin KD, Tyeklár Z. Bioinorganic Chemistry of Copper. Chapman & Hall; New York: 1993.
-
- Schindler S. Eur J Inorg Chem. 2000;2311–2326
-
- Itoh S, Tachi Y. Dalton Trans. 2006:4531–4538. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

