Lipopolysaccharide mobility in leaf tissue of Arabidopsis thaliana
- PMID: 21029320
- PMCID: PMC6640497
- DOI: 10.1111/j.1364-3703.2010.00638.x
Lipopolysaccharide mobility in leaf tissue of Arabidopsis thaliana
Abstract
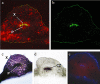
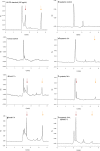
Bacterial lipopolysaccharides (LPS) are triggers of defence responses in plants, and induce local as well as systemic acquired resistance. Arabidopsis thaliana plants pretreated with LPS show an increased resistance to the virulent bacterial plant pathogen Pseudomonas syringae pv. tomato DC3000. To investigate the mobilization and transport of LPS in Arabidopsis leaves, fluorescently labelled LPS (Alexa Fluor® 488 conjugate) from Salmonella minnesota was used. Leaves were pressure infiltrated with fluorescein-labelled LPS and fluorescence microscopy was used to follow the movement and localization of LPS as a function of time. The observation of leaves 1 h after supplementation with fluorescein-labelled LPS revealed a fluorescent signal in the intercellular space. Capillary zone electrophoresis was used for the detection and analysis of the labelled LPS in directly treated leaves and systemic leaves. In addition, gel electrophoresis was used to confirm LPS mobilization. The results indicated that LPS mobilization/translocation occurs through the xylem from local, treated leaves to systemic, untreated leaves. Consequently, care should be taken when ascribing the observed biochemical responses and induced resistance from LPS perception as being uniquely local or systemic, as these responses might overlap because of the mobility of LPS in the plant vascular system.
© 2010 The Authors.Molecular Plant Pathology © 2010 BSPP and Blackwell Publishing Ltd.
Figures



Similar articles
-
Chitosan Oligosaccharide Induces Resistance to Pseudomonas syringae pv. tomato DC3000 in Arabidopsis thaliana by Activating Both Salicylic Acid- and Jasmonic Acid-Mediated Pathways.Mol Plant Microbe Interact. 2018 Dec;31(12):1271-1279. doi: 10.1094/MPMI-03-18-0071-R. Epub 2018 Oct 4. Mol Plant Microbe Interact. 2018. PMID: 29869942
-
IDL6-HAE/HSL2 impacts pectin degradation and resistance to Pseudomonas syringae pv tomato DC3000 in Arabidopsis leaves.Plant J. 2017 Jan;89(2):250-263. doi: 10.1111/tpj.13380. Epub 2017 Jan 16. Plant J. 2017. PMID: 27618493
-
N-cyanomethyl-2-chloroisonicotinamide induces systemic acquired resistance in arabidopsis without salicylic acid accumulation.Biosci Biotechnol Biochem. 2003 Feb;67(2):322-8. doi: 10.1271/bbb.67.322. Biosci Biotechnol Biochem. 2003. PMID: 12728993
-
Involvement of the salicylic acid signaling pathway in the systemic resistance induced in Arabidopsis by plant growth-promoting fungus Fusarium equiseti GF19-1.J Oleo Sci. 2013;62(6):415-26. doi: 10.5650/jos.62.415. J Oleo Sci. 2013. PMID: 23728333
-
Methyl Salicylate Glucosylation Regulates Plant Defense Signaling and Systemic Acquired Resistance.Plant Physiol. 2019 Aug;180(4):2167-2181. doi: 10.1104/pp.19.00091. Epub 2019 Apr 8. Plant Physiol. 2019. PMID: 30962291 Free PMC article.
Cited by
-
MAMP (microbe-associated molecular pattern) triggered immunity in plants.Front Plant Sci. 2013 May 16;4:139. doi: 10.3389/fpls.2013.00139. eCollection 2013. Front Plant Sci. 2013. PMID: 23720666 Free PMC article.
-
Activation of camalexin biosynthesis in Arabidopsis thaliana in response to perception of bacterial lipopolysaccharides: a gene-to-metabolite study.Planta. 2012 Jul;236(1):261-72. doi: 10.1007/s00425-012-1606-1. Epub 2012 Feb 18. Planta. 2012. PMID: 22350766
-
The Lipopolysaccharide-Induced Metabolome Signature in Arabidopsis thaliana Reveals Dynamic Reprogramming of Phytoalexin and Phytoanticipin Pathways.PLoS One. 2016 Sep 22;11(9):e0163572. doi: 10.1371/journal.pone.0163572. eCollection 2016. PLoS One. 2016. PMID: 27656890 Free PMC article.
-
Genetic requirements for infection-specific responses in conferring disease resistance in Arabidopsis.Front Plant Sci. 2022 Nov 29;13:1068438. doi: 10.3389/fpls.2022.1068438. eCollection 2022. Front Plant Sci. 2022. PMID: 36523630 Free PMC article.
-
Transcriptome analysis reveals key roles of AtLBR-2 in LPS-induced defense responses in plants.BMC Genomics. 2017 Dec 29;18(1):995. doi: 10.1186/s12864-017-4372-4. BMC Genomics. 2017. PMID: 29284410 Free PMC article.
References
-
- Bakker, P.A. , Pieterse, C.M. and Van Loon, L.C. (2007) Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology, 97, 239–243. - PubMed
-
- Beers, E.P. and McDowell, J.M. (2001) Regulation and execution of programmed cell death in response to pathogens, stress and developmental cues. Curr. Opin. Plant Biol. 4, 561–567. - PubMed
-
- Boller, T. and Felix, G. (2009) A renaissance of elicitors: perception of microbe‐associated molecular patterns and danger signals by pattern‐recognition receptors. Annu. Rev. Plant Biol. 60, 379–406. - PubMed
-
- Che, F.S. , Nakajima, Y. , Tanaka, N. , Iwano, M. , Yoshida, T. , Takayama, S. , Kadota, I. and Isogai, A. (2000) Flagellin from an incompatible strain of Pseudomonas avenae induces a resistance response in cultured rice cells. J. Biol. Chem. 275, 32 347–32 356. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

