Homodimerization of pokeweed antiviral protein as a mechanism to limit depurination of pokeweed ribosomes
- PMID: 21029321
- PMCID: PMC6640456
- DOI: 10.1111/j.1364-3703.2010.00640.x
Homodimerization of pokeweed antiviral protein as a mechanism to limit depurination of pokeweed ribosomes
Abstract
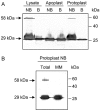
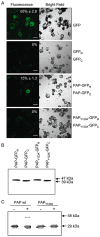
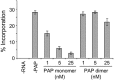
Ribosome inactivating proteins are glycosidases synthesized by many plants and have been hypothesized to serve in defence against pathogens. These enzymes catalytically remove a conserved purine from the sarcin/ricin loop of the large ribosomal RNA, which has been shown in vitro to limit protein synthesis. The resulting toxicity suggests that plants may possess a mechanism to protect their ribosomes from depurination during the synthesis of these enzymes. For example, pokeweed antiviral protein (PAP) is cotranslationally inserted into the lumen of the endoplasmic reticulum and travels via the endomembrane system to be stored in the cell wall. However, some PAP may retrotranslocate across the endoplasmic reticulum membrane to be released back into the cytosol, thereby exposing ribosomes to depurination. In this work, we isolated and characterized a complexed form of the enzyme that exhibits substantially reduced activity. We showed that this complex is a homodimer of PAP and that dimerization involves a peptide that contains a conserved aromatic amino acid, tyrosine 123, located in the active site of the enzyme. Bimolecular fluorescence complementation demonstrated that the homodimer may form in vivo and that dimerization is prevented by the substitution of tyrosine 123 for alanine. The homodimer is a minor form of PAP, observed only in the cytosol of cells and not in the apoplast. Taken together, these data support a novel mechanism for the limitation of depurination of autologous ribosomes by molecules of the protein that escape transport to the cell wall by the endomembrane system.
© 2010 The Authors. Molecular Plant Pathology © 2010 BSPP and Blackwell Publishing Ltd.
Figures



Similar articles
-
Pokeweed antiviral protein, a ribosome inactivating protein: activity, inhibition and prospects.Toxins (Basel). 2015 Jan 28;7(2):274-98. doi: 10.3390/toxins7020274. Toxins (Basel). 2015. PMID: 25635465 Free PMC article. Review.
-
Evidence for retro-translocation of pokeweed antiviral protein from endoplasmic reticulum into cytosol and separation of its activity on ribosomes from its activity on capped RNA.Biochemistry. 2005 Feb 22;44(7):2478-90. doi: 10.1021/bi048188c. Biochemistry. 2005. PMID: 15709760
-
Active center cleft residues of pokeweed antiviral protein mediate its high-affinity binding to the ribosomal protein L3.Biochemistry. 2001 Aug 7;40(31):9104-14. doi: 10.1021/bi002851p. Biochemistry. 2001. PMID: 11478877
-
Pokeweed antiviral protein regulates the stability of its own mRNA by a mechanism that requires depurination but can be separated from depurination of the alpha-sarcin/ricin loop of rRNA.J Biol Chem. 2002 Nov 1;277(44):41428-37. doi: 10.1074/jbc.M205463200. Epub 2002 Aug 8. J Biol Chem. 2002. PMID: 12171922
-
Pokeweed antiviral protein: its cytotoxicity mechanism and applications in plant disease resistance.Toxins (Basel). 2015 Mar 6;7(3):755-72. doi: 10.3390/toxins7030755. Toxins (Basel). 2015. PMID: 25756953 Free PMC article. Review.
Cited by
-
Pokeweed antiviral protein, a ribosome inactivating protein: activity, inhibition and prospects.Toxins (Basel). 2015 Jan 28;7(2):274-98. doi: 10.3390/toxins7020274. Toxins (Basel). 2015. PMID: 25635465 Free PMC article. Review.
-
Plant Ribosome-Inactivating Proteins: Progesses, Challenges and Biotechnological Applications (and a Few Digressions).Toxins (Basel). 2017 Oct 12;9(10):314. doi: 10.3390/toxins9100314. Toxins (Basel). 2017. PMID: 29023422 Free PMC article. Review.
-
Depurination of Brome mosaic virus RNA3 inhibits its packaging into virus particles.Nucleic Acids Res. 2011 Sep 1;39(16):7209-22. doi: 10.1093/nar/gkr383. Epub 2011 May 23. Nucleic Acids Res. 2011. PMID: 21609957 Free PMC article.
-
An N-terminal fragment of yeast ribosomal protein L3 inhibits the cytotoxicity of pokeweed antiviral protein in Saccharomyces cerevisiae.Toxins (Basel). 2014 Apr 11;6(4):1349-61. doi: 10.3390/toxins6041349. Toxins (Basel). 2014. PMID: 24732204 Free PMC article.
-
A Novel Cysteine Protease from Phytolacca americana Cleaves Pokeweed Antiviral Protein Generating Bioactive Fragments.Plants (Basel). 2025 Aug 7;14(15):2441. doi: 10.3390/plants14152441. Plants (Basel). 2025. PMID: 40805790 Free PMC article.
References
-
- Ago, H. , Kataoka, J. , Tsuge, H. , Habuka, N. , Inagaki, E. , Noma, M. and Miyano, M. (1994) X‐ray structure of a pokeweed antiviral protein, coded by a new genomic clone, at 0.23 nm resolution. A model structure provides a suitable electrostatic field for substrate binding. Eur. J. Biochem. 225, 369–374. - PubMed
-
- Baykal, U. and Tumer, N.E. (2007) The C‐terminus of pokeweed antiviral protein has distinct roles in transport to the cytosol, ribosome depurination and cytotoxicity. Plant J. 49, 995–1007. - PubMed
-
- Bonness, M.S. , Ready, M.P. , Irvin, J.D. and Mabry, T.J. (1994) Pokeweed antiviral protein inactivates pokeweed ribosomes; implications for the antiviral mechanism. Plant J. 5, 173–183. - PubMed
-
- Bradford, M.M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal. Biochem. 72, 248–254. - PubMed
-
- Carzaniga, R. , Sinclair, L. , Fordham‐Skelton, A.P. , Harris, N. and Croy, R.D.R. (1994) Cellular and subcellular distribution of saporins, type‐1 ribosome‐inactivating proteins, in soapwort (Saponaria officinalis L.). Planta, 194, 461–470.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

