OPRM1 gene variants modulate amphetamine-induced euphoria in humans
- PMID: 21029375
- PMCID: PMC3377371
- DOI: 10.1111/j.1601-183X.2010.00655.x
OPRM1 gene variants modulate amphetamine-induced euphoria in humans
Abstract
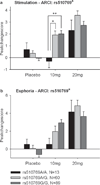
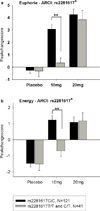
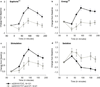
The μ-opioid receptor is involved in the rewarding effects of not only opioids like morphine but also psychostimulants like amphetamine. This study aimed to investigate associations between subjective response to amphetamine and genetic polymorphisms and haplotypes in the μ-opioid receptor including the exonic variant rs1799971 (Asp40Asn). One hundred and sixty-two Caucasian volunteers participated in three sessions receiving either placebo or d-amphetamine (10 and 20 mg). Associations between levels of self-reported Euphoria, Energy and Stimulation [Addiction Research Center Inventory 49-item questionnaire (ARCI-49)] after d-amphetamine ingestion and polymorphisms in OPRM1 were investigated. The intronic single nucleotide polymorphisms (SNPs) rs510769 and rs2281617 were associated with significantly higher ratings of Euphoria, Energy and Stimulation after 10 mg amphetamine. Feelings of Euphoria, Energy and Stimulation were also found to be associated with a two-SNP haplotype formed with rs1799971 and rs510769 and a three-SNP haplotype formed with rs1918760, rs2281617 and rs1998220. These results support the hypothesis that genetic variability in the μ-opioid receptor gene influences the subjective effects of amphetamine and may suggest new strategies for prevention and treatment of psychostimulant abuse.
© 2010 The Authors. Genes, Brain and Behavior © 2010 Blackwell Publishing Ltd and International Behavioural and Neural Genetics Society.
Conflict of interest statement
None.
Figures
References
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: 1994.
-
- Befort K, Filliol D, Decaillot FM, Gaveriaux-Ruff C, Hoehe MR, Kieffer BL. A single nucleotide polymorphic mutation in the human mu-opioid receptor severely impairs receptor signaling. J Biol Chem. 2001;276:3130–3137. - PubMed
-
- Brauer LH, de Wit H. Subjective responses to d-amphetamine alone and after pimozide pretreatment in normal healthy volunteers. Biol Psychiatry. 1996;39:26–32. - PubMed
-
- Caraco Y, Maroz Y, Davidson E. Variability in alfentanil analgesia may be attributed to polymorphism in the mu opioid receptor [abstract] Clin Pharmacol Ther. 2001;69:63.
-
- Crabbe JC, Jarvik LF, Liston EH, Jenden DJ. Behavioral responses to amphetamine in identical twins. Acta Genet Med Gemellol (Roma) 1983;32:139–149. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

