Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis
- PMID: 21029864
- PMCID: PMC2996235
- DOI: 10.1016/j.cell.2010.09.024
Endophilin functions as a membrane-bending molecule and is delivered to endocytic zones by exocytosis
Abstract
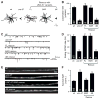
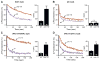
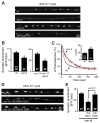
Two models have been proposed for endophilin function in synaptic vesicle (SV) endocytosis. The scaffolding model proposes that endophilin's SH3 domain recruits essential endocytic proteins, whereas the membrane-bending model proposes that the BAR domain induces positively curved membranes. We show that mutations disrupting the scaffolding function do not impair endocytosis, whereas those disrupting membrane bending cause significant defects. By anchoring endophilin to the plasma membrane, we show that endophilin acts prior to scission to promote endocytosis. Despite acting at the plasma membrane, the majority of endophilin is targeted to the SV pool. Photoactivation studies suggest that the soluble pool of endophilin at synapses is provided by unbinding from the adjacent SV pool and that the unbinding rate is regulated by exocytosis. Thus, endophilin participates in an association-dissociation cycle with SVs that parallels the cycle of exo- and endocytosis. This endophilin cycle may provide a mechanism for functionally coupling endocytosis and exocytosis.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




References
-
- Darcy KJ, Staras K, Collinson LM, Goda Y. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat Neurosci. 2006;9:315–321. - PubMed
-
- Dickman DK, Horne JA, Meinertzhagen IA, Schwarz TL. A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell. 2005;123:521–533. - PubMed
-
- Dittman J, Ryan TA. Molecular circuitry of endocytosis at nerve terminals. Annu Rev Cell Dev Biol. 2009;25:133–160. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

